
Brain-inspired Sensorimotor Robotic Platform
Learning in Cerebellum-driven Movement Tasks through a Cerebellar
Realistic Model
Claudia Casellato
1
, Jesus A. Garrido
2,3
, Cristina Franchin
1
, Giancarlo Ferrigno
1
,
Egidio D’Angelo
2,4
and Alessandra Pedrocchi
1
1
Politecnico di Milano, Dept of Electronics, Information and Bioengineering, Via G. Colombo 40, 20133 Milano, Italy
2
University of Pavia, Dept of Brain and Behavioral Sciences, Neurophysiology Unit, Via Forlanini 6, I-27100 Pavia, Italy
3
Consorzio Interuniversitario per le Scienze Fisiche della Materia (CNISM), Via Forlanini 6, I-27100 Pavia, Italy
4
IRCCS, Istituto Neurologico Nazionale C. Mondino, Brain Connectivity Center, Via Mondino 2, Pavia, I-27100, Italy
Keywords: Cerebellum, Learning, Vestibular-Ocular Reflex, Plasticity.
Abstract: Biologically inspired neural mechanisms, coupling internal models and adaptive modules, can be an
effective way of constructing a control system that exhibits a human-like behaviour. A brain-inspired
controller has been developed, embedding a cerebellum-like adaptive module based on neurophysiological
plasticity mechanisms. It has been tested as controller of an ad-hoc developed neurorobot, integrating a 3
degrees of freedom serial robotic arm with a motion tracking system. The learning skills have been tried out,
designing a vestibular-ocular reflex (VOR) protocol. One robot joint was used to get the desired head turn,
while another joint displacement corresponded to the eye motion, which was controlled by the cerebellar
model output, used as joint torque. Along task repetitions, the cerebellum was able to produce an
anticipatory eye displacement, which accurately compensated the head turn in order to keep on fixing the
environmental object. Multiple tests have been implemented, pairing different head turn with object motion.
The gaze error and the cerebellum output were quantified. The VOR was accurately tuned thanks to the
cerebellum plasticity. The next steps will include the activation of multiple plasticity sites evaluating the
real platform behaviour in different sensorimotor tasks.
1 INTRODUCTION
Biologically inspired neural mechanisms can be an
effective way of constructing a control system that
exhibits a human-like behavior. In the framework of
distributed motor control, connecting brain-inspired
kinematic and dynamic models, human-like
movement planning strategies and adaptive neural
systems inside the same controller is a very
challenging approach, bridging neuroscience and
robotics.
Motor learning is obviously necessary for
complicated movements such as playing the piano,
but it is also important for calibrating simple
movements like reflexes, as parameters of the body
and environment change over time. Cerebellum-
dependent learning is demonstrated in different
contexts (Boyden et al., 2004; van der Smagt, 2000),
such as multiple forms of associative learning,
where the learning is based on the stimulus-response
association. Eye blinking conditioning, saccadic eye
movements, vestibular ocular reflex and reaching
arm movements are well-known examples of these
mechanisms (Donchin et al., 2012).
The Marr-Albus and Ito models propose that
changes in the strengths of parallel fiber–Purkinje
cell synapses could store stimulus-response
associations by linking inputs with appropriate
motor outputs, following a Hebbian learning
approach. Error-based learning and predictive
outputs are working principles of these cerebellum
models (Marr, 1969; Albus, 1971; Ito, 2006).
We have built up a real human-like sensorimotor
platform with cerebellar-like learning skills. The
sensory systems are integrated, monitoring the
environment and the muscular-skeletal system state.
Based on the neurophysiology of the distributed
neuromotor control system, the robotic control has
568
Casellato C., A. Garrido J., Franchin C., Ferrigno G., D’Angelo E. and Pedrocchi A..
Brain-inspired Sensorimotor Robotic Platform - Learning in Cerebellum-driven Movement Tasks through a Cerebellar Realistic Model.
DOI: 10.5220/0004659305680573
In Proceedings of the 5th International Joint Conference on Computational Intelligence (SSCN-2013), pages 568-573
ISBN: 978-989-8565-77-8
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)
been designed as a real-time coupling of multiple
neuronal structures and mechanisms; the cerebellum
model, based on the plasticity principles ascribed to
the synapses between parallel fibers and Purkinje
cells, is expected to learn throughout the task
repetitions (Casellato et al., 2012).
One protocol stressing the cerebellum role has
been designed and implemented: the vestibular-
ocular reflex (VOR). The VOR produces eye
movements which aim at stabilizing images on the
retina during head movement. The VOR tuning is
ascribed mainly to the cerebellum loop, in particular
to the cerebellar flocculus which creates an
inhibitory loop in the VOR circuit. There are a lot of
evidences of its role from lesions, pharmacological
inactivation and genetic disruption studies (Burdess,
1996). The learning is based on the temporal
association of the two stimuli, head turn and motion
of retinal image, i.e. the system learns that one
stimulus will be followed by another stimulus and a
consequent predictive compensatory response is
gradually produced and accurately tuned.
2 METHODS
2.1 Robotic Platform
A flexible, not cumbersome and manoeuvrable
robotic platform has been built and its controller has
been developed in Visual C++.
The main robot is a Phantom Premium 1.0
(SensAble
TM
), with 3 rotational Degrees of Freedom
(DoFs). It is equipped with digital encoders at each
joint and it can be controlled with force and torque
commands. It connects to the PC via the parallel port
interface. It is integrated with a motion capture
device, a VICRA-Polaris (NDI
TM
), which is an
optical measurement system acquiring marker-tools
at 20 Hz. Another robotic device (Phantom Omni,
SensAble
TM
) is included into the platform so as to
impose object motion during the protocol
performance. The controller has been developed
exploiting the OpenHaptics toolkit (SensAble
TM
)
and the Image-Guided Surgery Toolkit (IGSTK).
The robotic DoFs are controlled through torque
signals, by exploiting the low-level access provided
by the Haptic Device Application Programming
Interface (HDAPI). For stability, this control loop
must be executed at a consistent 1 kHz rate; in order
to maintain such a high update rate, the servo loop is
executed in a separate, high-priority thread
(HDCALLBACKS). For the motion tracking system
integration, the IGSTK low-level libraries
(http://www.igstk.org/), whose architecture is based
on Request-Observer-pattern, are used. Each desired
tool is identified by a .rom file, which defines the
unique geometry of the reflective markers
composing the tool itself.
In our configuration, wireless passive tools have
been placed in correspondence of the robotic end-
effector (gaze) and of the objects of interest in the
environment. An a-priori calibration procedure
allows to identify the constant roto-translation
between the reference system of the tracking device
(Visual system) and of the robot (Proprioceptive
system), thus it represents a Body/Eye calibration.
2.2 Cerebellum Model
The cerebellar system hereby implemented and
embedded into the whole control system is based on
the model proposed in (Garrido et al., submitted). It
takes into account the major functional hypotheses
that each cerebellar layer endows, modeling Mossy
Fibers (MF), GRanular layer (GR), Purkinje Cell
layer (PC) and Deep Cerebellar Nuclei (DCN).
These cerebellar layers have been interconnected, as
shown in Figure 1, where PF (Parallel Fibers) are the
axons of GR cells and the CF are the Climbing
Fibers coming from the Inferior Olive (IO). This
model implements three plasticity mechanisms at
different synapses: PF→PC (w), PC→DCN (b) and
MF→DCN (v). In previous models, the GR layer
has been suggested to generate non-recurrent states
after the stimulus onset in eyeblink-conditioning
tasks (Yamazaki and Tanaka, 2007). The large
number of granular cells (and then of their axons,
i.e. PF) guarantees a reliable pattern separation,
which means that similar input patterns would be
sparsely re-encoded into largely not-overlapping
populations of GR activity. Climbing fibers carry the
error signal, generating complex spikes on PC. A
state-error correlator emulates the Purkinje cell
operability, driving the PF→PC long-term plasticity.
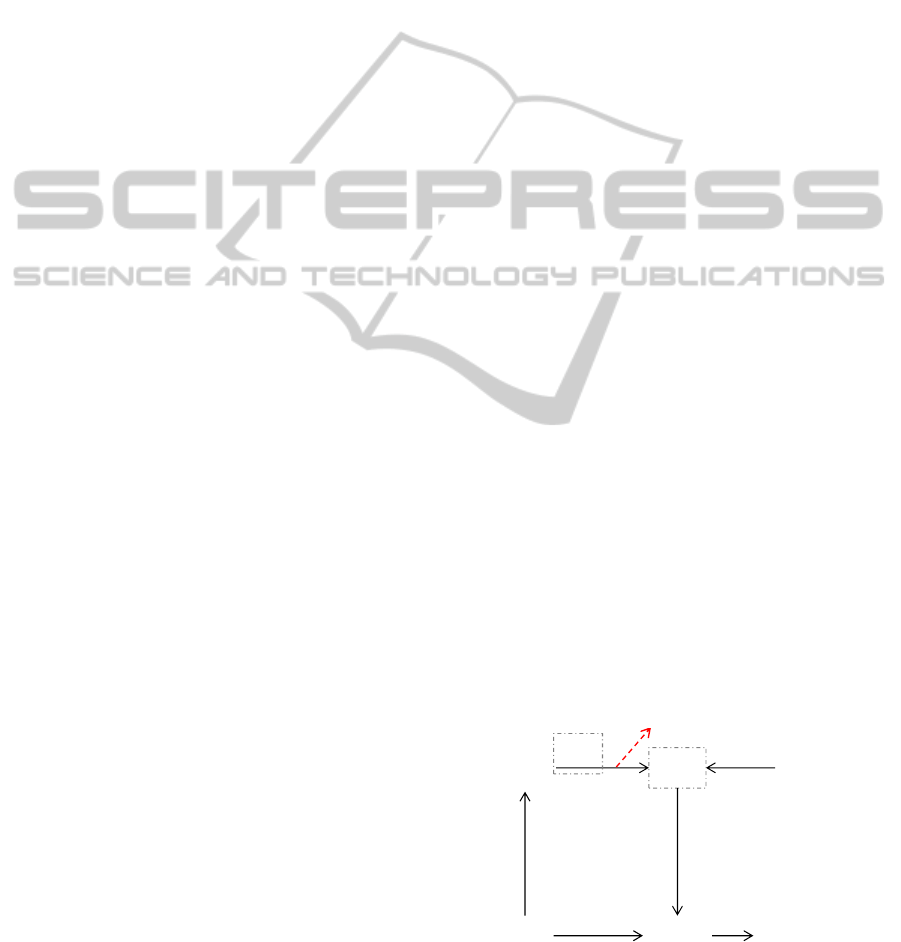
Figure 1: Scheme of the cerebellar network.
MF
GR
PC
PF
IO
CF
DCN
W
b
v
z(t)=
v∙(MF(t))‐ b∙(PC(t))
Brain-inspiredSensorimotorRoboticPlatform-LearninginCerebellum-drivenMovementTasksthroughaCerebellar
RealisticModel
569

Finally, an adder/subtractor module receives the
inputs coming from the mossy fibers (multiplied by
the MF→DCN synaptic weight v) and subtracts the
signal coming from the cerebellar cortex (multiplied
by its own synaptic weight b). Indeed, the
MF→DCN connections are excitatory, while the
PC→DCN ones are inhibitory. In the cerebellum
model used in this work (Figure 1), only one
plasticity site was taken into account: the long-term
depression (LTD) and long-term potentiation (LTP)
at cerebellar cortex (PF→PC); thus, the PC activity
changes along time and along repetitions (PC(t))
depending on the tuneable strength at the synapses
with the PF (w). The synapses strengths v and b are
set constant equal to 1. Thus, the DCN output is
maximum (z=1) when the Purkinje cells do not fire
(PC(t)=0), i.e. the PF→PC weights are depressed
(LTD); whereas the DCN output is minimum (z=0)
when the Purkinje cells are maximally activated
(PC(t)=1), i.e. the PF→PC weights are potentiated
(LTP). LTD is driven by CF, in particular it is
proportionally induced by the frequency of the
generated complex spikes; whereas LTP is
constantly produced when an input activity is
present but in absence of CF stimulation related to
this activity (unsupervised learning). The plasticity
rule on w is defined as follows:
∆
→
1
∙
0
where Δw
PF
j
-PC
i
(t) represents the weight change
between the j-th parallel fiber and the target PC
associated to the muscle (agonist or antagonist), ԑ
i
is
the current activity coming from the associated
climbing fiber (which represents the normalized
error along the executed head-eye movement),
LTP
max
and LTD
max
are the maximum LTP and LTD
values and α is the LTP decaying factor. LTP
max
and
LTD
max
are set 0.015 and 0.15 respectively and α =
1000, to avoid early plasticity saturation.
The somatotopic approach is kept at level of IO,
PC and DCN: a group of IO carries information
about the error (positive and negative separately) of
a specific involved DoF and projects on a
corresponding group of PC. They themselves project
on a corresponding group of DCN, which thus
produce an agonist or antagonist motion (positive
and negative) for each specific controlled DoF.
2.3 Protocol: VOR
The stimulus is the head turn, which is produced by
rapidly imposing a motion to the joint 2 of the
robotic device, with a pre-defined joint time-profile,
occurring in a head-fixed reference system (fixed
robotic reference system). The gaze is defined as the
orientation of the second link of the robot, i.e. the
one linking joint 2 and joint 3.
The vestibular sensory input is used by the GR
layer to generate the system state.
The CF carry the visual error, i.e. the image
retinal slip, computed with data from the optical
tracking system. Assumed that the goal is to fix an
environmental object, this visual error is computed
as the disalignment angle with respect to the stable
condition before head turn, where the gaze direction
and the object center are aligned. Since the
acquisition frequency of the tracking system, the
retinal slip is more delayed or not strictly
synchronized with the faster vestibular information,
which is physiologically meaningful.
During the head turn, the only controller acting
on joint 3 (compensatory eye turn) is the cerebellum,
so as to be fully neurophysiologically plausible.
Indeed, since the required rapid reflexive response to
compensate for head motion, the inaccurate and
delayed feedback cannot be activated. It is worthy to
note that the robotic device was used so as to get the
two involved joints (joints 2 and 3) moving on a
horizontal plane, where the gravity has not to be
counterbalanced. The protocol and the set-up are
shown in Figure 2.
Figure 2: The VOR protocol. A: stable condition. B: head
turn leading to an image slip. C: head turn and
compensatory eye movement. D: the set-up: Phantom
Premium with the optical tool on the end-effector;
Phantom Omni with the object-tool. The green laser is
attached parallel to the second link (i.e. the gaze direction)
to highlight the gaze point on the environmental scene.
In order to quantify the VOR performance, the
gain at maximum vestibular stimulus has been
computed, as absolute ratio between eye turn and
head turn (angles) at the maximum achieved head
turn. Different sequences of repetitions were tested,
in order to quantify the modulation of VOR with
different stimuli presentation.
• Test 1 –VOR Calibration
We have designed a sequence of 130 trials. In the
first 110 repetitions, a head turn of 22° with respect
IJCCI2013-InternationalJointConferenceonComputationalIntelligence
570
to the rest head-tilt was imposed with a speed equal
to 5.5°/s (head turn duration = 4 s). During the last
20 trials, the head turn was halved (11°, at 2.75°/s).
Each trial lasted 4.5 seconds, thus including a
stabilization phase between consecutive trials (0.5
s), where the head turn did not occur. The eye
motion was driven only by the cerebellum output
(joint 3 torque), with a constant gain equal to 100.
• Test 2: Head Motion + Object Motion
The head motion was paired with additive image
motion; it means that the image motion was due not
only to the head turn but even to the real object
displacement (Boyden et al., 2004). We have tested
a sequence of 110 trials where the object motion was
synchronously added to the head rotation (same
onset instant). The head turn was as in Test 1 (22° in
4 seconds). The object, attached to the Phantom
Omni, was moved at a speed equal to 4 cm/s. Three
conditions were created; the first one was defined as
a standard VOR calibration; the second condition
with the object moving in the same direction as the
head and then the third condition with the object
moving in the opposite direction than the head
rotation.
3 RESULTS
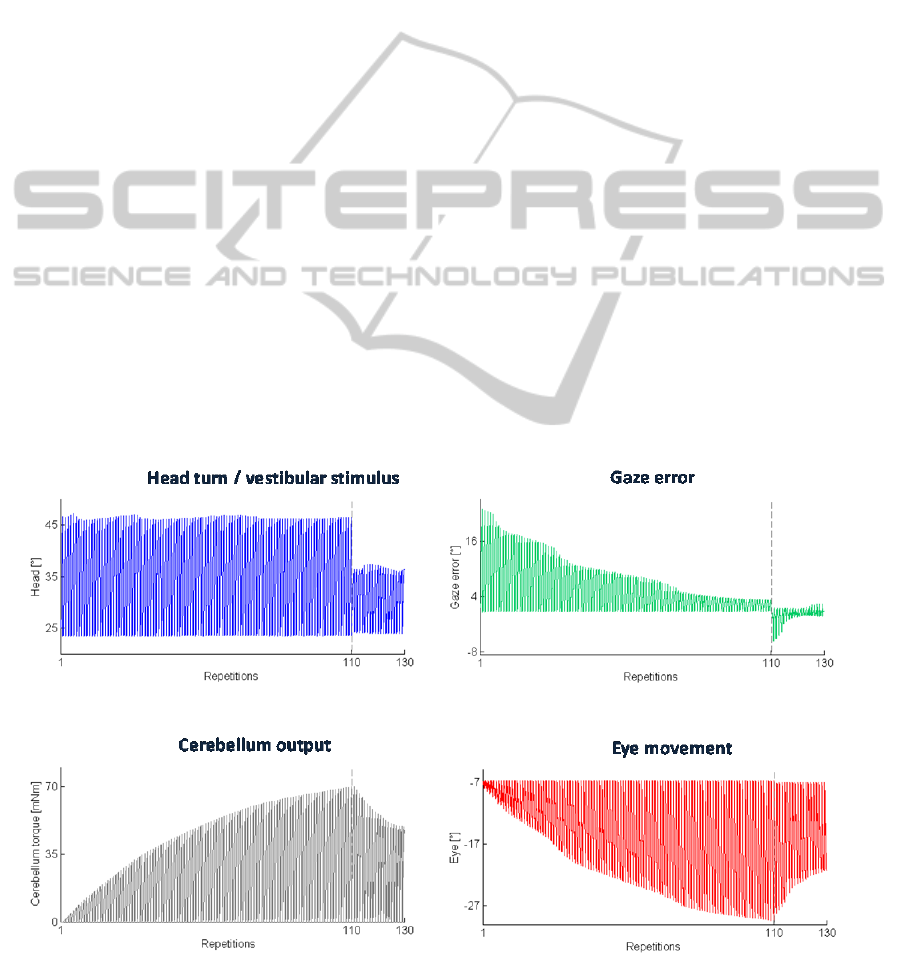
• Test 1 – VOR Calibration
Figure 3 shows the results of these tests.
In the first trials of the task sequence, the VOR is
poorly calibrated, thus head movement results in
image motion on the retina, which would mean
blurred vision. Indeed, in these first trials, the gaze
error reaches values higher than 16° when the head
is maximally turned. Along task repetitions, the
cerebellum-driven motor learning adjusts the VOR
to produce more accurate eye motion, thus reducing
and stabilizing the gaze error.
In the last 20 trials (2
nd
condition), where the
head rotation amplitude is reduced, the first
repetitions are characterized by an eye
overcompensation, as learnt during the previous
condition; it means that the eyes turn too much with
respect to the head motion, overcoming the object.
Very rapidly, the VOR is re-tuned, stabilizing back
the error at the minimum level. From the zoom at the
right-top of the figure, it can be marked that the
shape of the gaze error changes between the two
conditions, since the onset of the head motion is the
same but the head is rotating slower and it reaches a
less turn from the initial head-tilt; thus the
cerebellum-driven eye motion starts correctly but
then it overcompensates the head motion. That is
adjusted within few repetitions of this 2
nd
condition.
Figure 3: Test 1 – VOR Calibration. Top-left: the actual joint 2; top-right: the gaze error; down-left: the cerebellar output
(DCN activity); down-right: the eye movement produced by the cerebellum output.
Brain-inspiredSensorimotorRoboticPlatform-LearninginCerebellum-drivenMovementTasksthroughaCerebellar
RealisticModel
571
The cerebellum output (already multiplied to the
gain, thus equal to the torque provided to joint 3
actuator) and the produced eye motion increase.
Then, in the 2
nd
condition where the head rotation
amplitude is reduced, VOR needs to be decreased;
thus, the cerebellar activity is modulated until it
reaches an analogous value as at the end of the first
condition. The decreasing process is faster than the
increasing one.
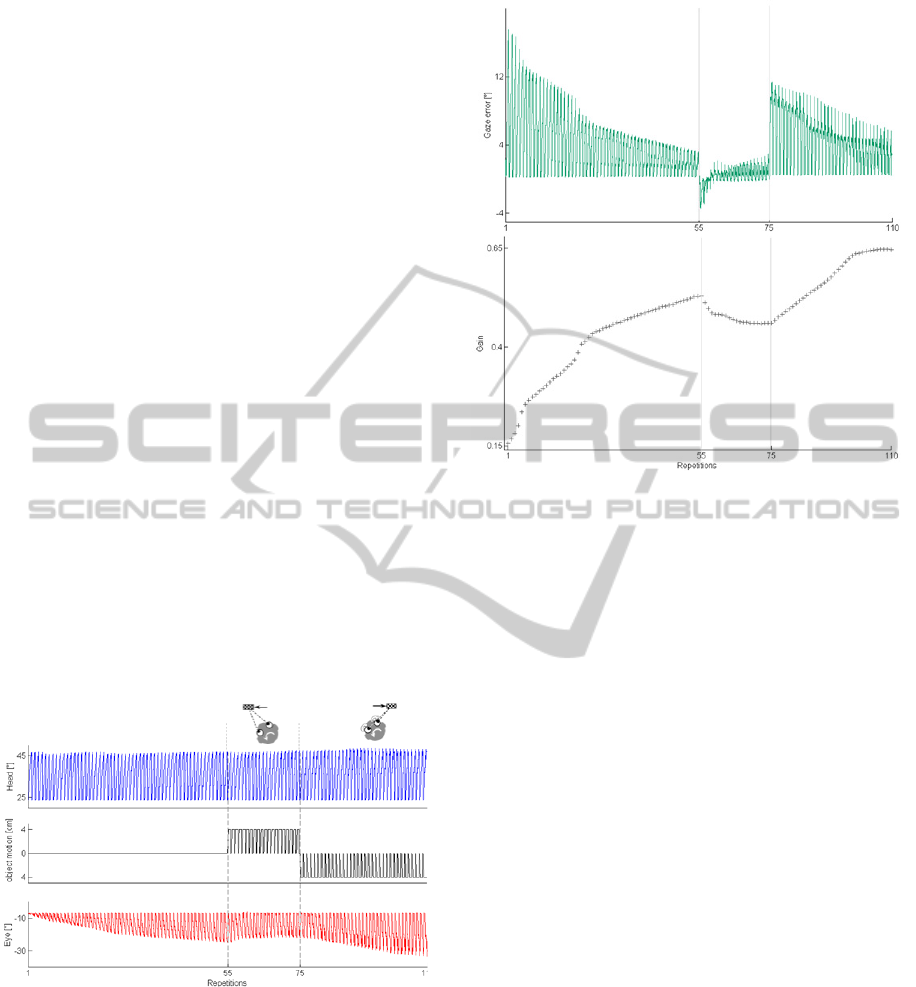
• Test 2: Head Motion + Object Motion
The sequence of 110 trials where the head turn is
constant and the object motion, when occurs, starts
together with the head motion is depicted in Figure
4. After a VOR calibration compensating only for
head turn, the additive object motion represents a
gain-down and a gain-up stimulus (2
nd
and 3
rd
conditions, respectively). Since, as demonstrated in
Test 1, the VOR decreasing is faster, the 2
nd
condition is made up of less repetitions than 3
rd
condition.
Figure 5 is focused on the gaze error and the
gain parameter, which is tuned during the repetitions
in each condition. Unlike Test 1, here the stable gain
that is achieved at the end of each condition is not
the same; indeed, the object motion induces a
modulation of the needed eye motion, even if the
head turn has the same amplitude and duration
across all conditions. Thus, the gain is modified
(gain-down and gain-up).
Figure 4: Test 2 - head motion + object motion. The first
row shows the head angle; the second row depicts the
object movement (by Phantom Omni motion); the third
row represents the produced eye compensatory eye
movement; in the three conditions: the VOR calibration
with fixed object, the VOR gain-down and the gain-up
inducing object motion.
Figure 5: The gaze error and the gain parameter in the task
sequence with gain-down and gain-up stimuli.
4 DISCUSSION
The developed integrated robotic platform with its
controller is able to neurophysiologically reproduce
representative cerebellum-driven motor behaviors.
The cerebellar model can be viewed as a “black
box” associative memory, whose function is
determined by how its inputs and outputs are
connected in the system that is embedded in.
The VOR uses a head turn signal sensed by the
vestibular system to drive an implicit feedfoward
compensation scheme. Purkinje cells activity can
properly tune eye movements.
In the first test about VOR calibration, the two
conditions in row show a sort of generalization of
learning, tuning the VOR response depending on the
features of the head turn stimulus. In
neurophysiological terms, the generalization process
should be based on an overlap of neuronal
activations, i.e. neurons active at low vestibular
stimuli are a subset of those active at higher stimuli.
The second test highlights the VOR tuning with
additive perturbations due to object displacement.
We found that the gain-down trials show a faster
learning rate than the gain-up trials. This asymmetry
is due to the different mechanisms; indeed, since the
system was set to inhibit the DCN activity at the
start of the learning process, increasing in VOR
involves only the LTD mechanism (reduction of PC
IJCCI2013-InternationalJointConferenceonComputationalIntelligence
572

activity and thus increase of DCN activity). On the
other hand, the need of decreasing VOR and the
presence of overcompensation enable
simultaneously the reduction of agonist DCN
activity (PF-PC LTP) and the increase of antagonist
DCN activity (PF-PC LTD). Thus, during the VOR
gain-down, the stiffness of the muscle is temporally
increased and, after some time, it is reduced to the
minimal level.
In summary, a simple model with parallel,
sparsely coded channels, and with a single plasticity
mechanism that alters a subset of these channels, can
go a long way in explaining the general capacity of
motor learning in the VOR to exhibit specificity for
the particular stimuli present during training.
Learning, modulation and extinction proprieties
emerge.
According to the plasticity distribution at
multiple synaptic sites (Gao et al., 2012), the next
steps will be focused on the activation of the other
plasticity rules of the cerebellar model, expecting a
more stable and more accurate learning. The
modulation of these connectivities (MF→DCN and
PC→DCN) should lead to a learning generalization,
which will be tested through multiple tasks, such as
force-field paradigm in multi-joint reaching and
associative protocols such as eye blinking classical
conditioning task (Hwang and Shadmehr, 2005;
Yamamoto et al., 2007; Hoffland et al., 2012).
Moreover, for a more realistic computational
scenario, the sensorimotor platform will embed the
spiking version of this developed multiple-plasticity
cerebellar model (Luque et al., 2011).
5 CONCLUSIONS
The developed platform, a real neuro-robot able to
interact with the environment in multiple forms, is a
flexible and versatile test bed to concretely interpret
specific features of functional biological models, in
terms of neural connectivity, plasticity mechanisms
and functional roles into different closed-loop
sensorimotor tasks. In particular, the focus is on the
most CNS plastic structure, the cerebellum.
ACKNOWLEDGEMENTS
This work has been supported by the EU grant
REALNET (FP7-ICT-270434).
REFERENCES
Albus, J.S. (1971). A Theory of Cerebellar Function. Math
Biosci., 10, 25–61.
Boyden, E.S., Katoh, A., Raymond, J.L. (2004).
Cerebellum-dependant Learning: The Role of Multiple
Plasticity Mechanisms. Annu Rev Neurosci., 27, 581–
609.
Burdess, C. (1996). The Vestibulo-Ocular Reflex:
Computation in The Cerebellar Flocculus. Retrieved
(n.d.) from hxxp://bluezoo.org/vor/vor.pdf.
Casellato, C., Pedrocchi, A., Garrido, J.A., Luque, N.R.,
Ferrigno, G., D’Angelo, E., Ros, A. (2012). An
Integrated Motor Control Loop of a Human-like
Robotic Arm: Feedforward, Feedback and
Cerebellum-based Learning. Biomedical Robotics and
Biomechatronics (BioRob), 2012 June 24-27 4th IEEE
RAS & EMBS International Conference, no., 562-567.
doi: 10.1109/BioRob.2012.6290791.
Donchin, O., Rabe, K., Diedrichsen, J., Schoch, B.,
Gizewski, E.R., Timmann, D. (2012). Cerebellar
Regions Involved in Adaptation to Force Field and
Visuomotor Perturbation. J Neurophysiol., 107, 134–
147.
Gao, Z., van Beugen, B.J., De Zeeuw, C.I. (2012).
Distributed Synergistic Plasticity and Cerebellar
Learning. Nat Rev Neurosci., 13, 619-35.
Garrido, J.A., Luque, N.R., D’Angelo, E., Ros, E.
Distributed cerebellar plasticity implements adaptable
gain control in a manipulation task: a closed-loop
robotic simulation. Submitted to Front. Neural
Circuits.
Hwang, E.J., Shadmehr, R.J. (2005). Internal Models of
Limb Dynamics and The Encoding of Limb State.
Neural Eng., 2(3), 266-78.
Hoffland, B.S., Bologna, M., Kassavetis, P., Teo, J.T.H.,
Rothwell, J.C., Yeo, C.H., van de Warrenburg, B.P.,
Edwards, M.J. (2012). Cerebellar Theta Burst
Stimulation Impairs Eyeblink Classical Conditioning.
J Physiol., 590(4), 887-897.
Ito M. (2006). Cerebellar Circuitry as a Neuronal
Machine. Prog Neurobiol., 78, 272–303.
Luque, N.R., Garrido, J.A., Carrillo, R.R., Coenen, O.J.,
Ros, E. (2011). Cerebellar Input Configuration
Toward Object Model Abstraction in Manipulation
Tasks. Neural Networks, IEEE Transactions on, 22(8),
1321-1328.
Marr, D. (1969). A Theory of Cerebellar Cortex. J
Physiol., 202(2), 437–470.
van der Smagt, P. (2000). Benchmarking Cerebellar
Control. Robotics and Autonomous Systems, 32, 237-
251.
Yamamoto, K., Kawato, M., Kotosaka, S., Kitazawa, S.
(2007). Encoding of Movement Dynamics by Purkinje
Cell Simple Spike Activity during Fast Arm
Movements under Resistive and Assistive Force Field.
J Neurophysiol., 97(2), 1588-99.
Yamazaki, T., Tanaka, S. (2007). The cerebellum as a
liquid state machine. Neural Networks, 20(3), 290-
297.
Brain-inspiredSensorimotorRoboticPlatform-LearninginCerebellum-drivenMovementTasksthroughaCerebellar
RealisticModel
573
