
Perfusion and Specific Radioprobes for Cardiac Imaging
Filipa Mendes, Lurdes Gano, Célia Fernandes, António Paulo and Isabel Santos
Radiopharmaceutical Sciences Group, IST/CTN, Instituto Superior Técnico,
Universidade Técnica de Lisboa, Estrada Nacional 10, km 139,7, 2695-066 Bobadela LRS, Portugal
Keywords: Nuclear Imaging, Molecular Imaging, Perfusion Agents, Targeted Radioprobes, Technetium, Fluorine.
Abstract: Major advancements have been made in treating cardiovascular disease. However, improving diagnosis is
crucial, because the detection of the early stages of disease would allow preventative approaches therapy.
Myocardial perfusion imaging is in clinical use for decades and is an effective tool for diagnosis, and long-
term follow-up of patients with suspected or known coronary artery disease. The technetium-based agents,
99mTc-sestamibi and 99mTc-tetrofosmin, are widely used myocardial blood flow tracers. However, since
both present drawbacks in their biodistribution properties, there is now resurgence in the study of both
neutral and cationic technetium agents to further improve the characteristics of perfusion
radiopharmaceuticals. Despite all the success of perfusion imaging, a unique strength of nuclear imaging is
its ability to provide tools for imaging processes at molecular and cellular levels in intact organisms under a
wide variety of physiologic conditions. Advances in new specific imaging agents that identify myocardium
injury and cellular dysfunction may contribute to the improvement of diagnosis and eventually better
therapeutic approaches. In this communication, we will review perfusion agents and their biological
mechanism of uptake. We will also discuss examples of target-specific radiopharmaceuticals for cardiac
imaging, including advances in pre-clinical imaging approaches.
1 MYOCARDIAL PERFUSION
IMAGING
In the past few decades, major improvements have
been made in treating some types of cardiovascular
disease. However, new treatment options are
urgently needed for all types of cardiovascular
disease. Moreover, improving diagnosis is crucial,
because by detecting the early stages of disease, the
focus of therapy could be shifted from treatment to
prevention.
Myocardial perfusion imaging has been in
clinical use for over 30 years, serving as an
effective, reliable, and relatively simple tool for
diagnosis, risk stratification, and long-term follow-
up of patients with suspected or known coronary
artery disease (Notghi and Low, 2011). Thallium-
201 chloride was the first pharmaceutical to be
widely used clinically for imaging myocardial
perfusion. Because of its relatively long half-life and
low energy X-ray emission, it is not the ideal agent
for imaging, giving a relatively large radiation dose
with lower image quality than technetium agents. It
enters the cells via the Na/K-ATPase, and is
redistributed fairly rapidly.
Technetium-based agents,
99m
Tc-sestamibi
(Cardiolite) and
99m
Tc-tetrofosmin (Myoview), are
now widely used myocardial blood flow tracers
(Figure 1). These perfusion agents have with
minimal redistribution, better imaging characteristics
and less radiation to the patient.
99m
Tc-sestamibi
enters the cell via a passive pathway due to its
lipophilicity and accumulates in the mitochondria in
response to the physiologically negative
mitochondrial and plasma membrane potentials. Due
to their elevated number of mitochondria, the heart,
muscles, liver and kidneys present a high uptake of
this radiopharmaceutical. Because the in vivo
behavior of
99m
Tc-tetrofosmin demonstrates
similarities with
99m
Tc-sestamibi it was initially
suggested that the mechanism determining cellular
distribution was also similar. The first studies
indicated that the uptake is through a metabolism-
dependent process, most likely by potential-driven
transport of the lipophilic cation. However,
subsequently it was shown that inhibition of the
Na+/K+ ATPase, partly inhibited the uptake of
79
Mendes F., Gano L., Fernandes C., Paulo A. and Santos I..
Perfusion and Specific Radioprobes for Cardiac Imaging.
DOI: 10.5220/0004664300790083
In Proceedings of the International Congress on Cardiovascular Technologies (VisualCardio-2013), pages 79-83
ISBN: 978-989-8565-78-5
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)
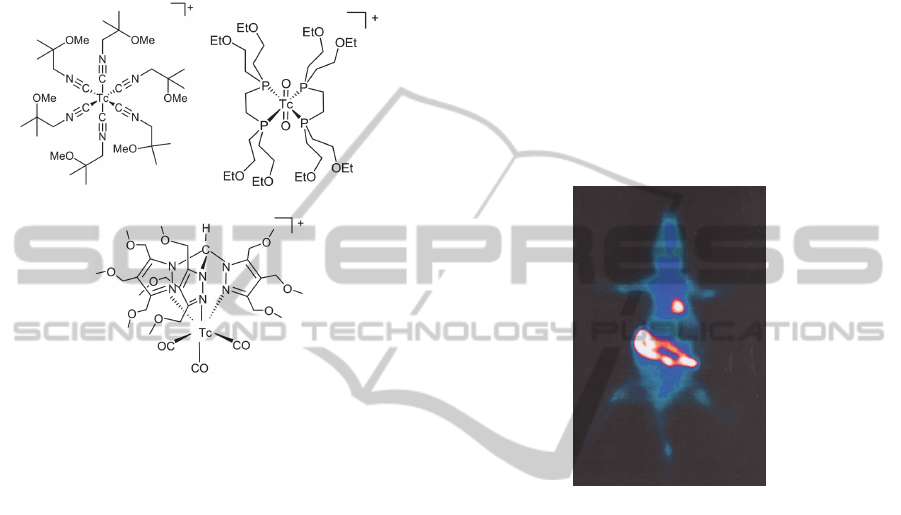
99m
Tc-tetrofosmin indicating that lipophilicity is not
the only factor involved in the cellular uptake.
Moreover, this agent appears to be more associated
with the cytosol than with mitochondria.
Nevertheless, it is consensual that
99m
Tc-tetrofosmin
uptake depends on both cell membrane and
mitochondrial potentials.
(1) (2)
(3)
Figure 1: Structures of
99m
Tc-sestamibi (1),
99m
Tc-
tetrofosmin (2) and
99m
Tc-TMEOP (3).
For the foreseeable future, myocardial perfusion
imaging will continue to be used for assessment of
ischaemia. However, both Cardiolite and Myoview
present biodistribution properties that suffer from
well-known drawbacks, the most important being
the high liver uptake, which can interfere in the
analysis of cardiac imaging, particularly of the
inferior left ventricular wall (
Germano, et al., 1994;
Kailasnath and Sinusas, 2001; Kapur, et al., 2002;
Llaurado, 2001; Parker, 2001)
. As a consequence, there
is now a resurgence and development of both neutral
and cationic technetium agents to further improve
the characteristics of perfusion radiopharmaceuticals
(Hatada et al., 2004); (Kim et al., 2008); (Liu, 2007);
(Liu et al., 2010).
Recently we were able to identify a new class of
organometallic complexes based on
tris(pyrazolyl)methane as lead structure (Garcia et
al., 2009); (Santos and Correia, 2005); (Maria et al.,
2007). This type of complexes has a cationic
character and they are stable both in vitro and in
vivo. In particular, we discovered that the
tricarbonyl complex
99m
Tc-TMEOP (Figure 1)
exhibited high heart uptake and biodistribution
properties suitable for myocardial imaging (Maria et
al., 2009); (Goethals et al., 2010) (Figure 2).
Data collected so far suggest that the
pharmacokinetic profile of
99m
Tc-TMEOP may
allow high quality imaging early after tracer
injection. Biodistribution and cardiac pinhole-gated
SPECT imaging studies in rats showed that
99m
Tc-
TMEOP has a cardiac uptake comparable to 99mTc-
sestamibi and
99m
Tc-tetrofosmin, but has a
significantly faster liver clearance (Goethals, et al.,
2010). At 40 min post injection, the heart/liver ratio
of
99m
Tc-TMEOP is twice that of
99m
Tc-sestamibi
and
99m
Tc-tetrofosmin (6.98±1.66, 2.48±0.30 and
2.66±0.40, respectively). Altogther, the data
collected so far suggest that the pharmacokinetic
profile of
99m
Tc-TMEOP may allow high quality
imaging early after tracer injection.
Figure 2: Planar image of a rat administered with
99m
Tc-
TMEOP, at 60 min pi. Image was performed in supine
position and acquired using a 128×128 matrix in a GE
gamma camera. Adapted from (Mendes et al., 2012).
Therefore, to get a better insight on the in vivo
behaviour of
99m
Tc-TMEOP, its mechanisms of
myocardial uptake and excretion have been
investigated. Our results indicate that the heart
uptake of
99m
Tc-TMEOP is related to its
accumulation in the mitochondria due to the
negative plasma and mitochondrial transmembrane
potentials (Mendes et al., 2012).
It is well know that cancer cells and tumours also
maintain a more negative potential owing to
increased metabolic requirements, and as a result,
there is an increased accumulation of
99m
Tc-
sestamibi,
99m
Tc-tetrofosmin and
99m
Tc-TMEOP in
malignant tumours. This feature permits the use of
these radiotracers for imaging cancers of the breast,
lung, brain and parathyroid adenomas (reviewed in
Mendes et al., 2011).
Despite all the success of perfusion imaging, a
unique strength of nuclear imaging is its ability to
provide tools for imaging biochemical and metabolic
CARDIOTECHNIX2013-InternationalCongressonCardiovascularTechnologies
80
processes and receptor and transporter functions at
molecular and cellular levels in intact organisms
under a wide variety of physiologic conditions.
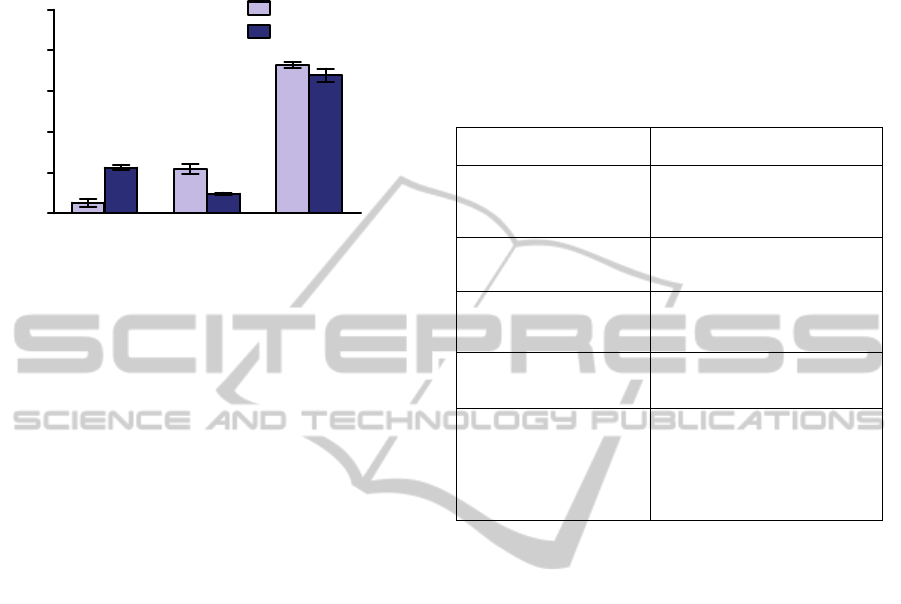
C
yt
osol
F
ragm
ent
s
M
i
tocho
nd
ria
0
20
40
60
80
100
99m
Tc-TMEOP
99m
Tc-Sestamibi
% total recovered
Figure 3: Subcellular distribution of
99m
Tc-TMEOP and
99m
Tc-Sestamibi in isolated rat's heart tissue. Adapted
from (Mendes et al., 2012).
2 CARDIAC MOLECULAR
IMAGING
Molecular imaging studies are shedding important
light on the cellular and molecular biology
underlying important cardiovascular diseases
(Osborn and Jaffer, 2012). Therefore within the field
of cardiovascular medicine, potential applications of
molecular imaging include the analysis of vulnerable
plaques, heart failure, neurohormonal dysfunction,
myocardial metabolism, stem cell engraftment,
protein–protein interactions, and angiogenesis.
(Table 1).
Myocardial Pathology. Metabolic adaptation
probably represents one of the earliest responses to
myocardial ischemia. The application of a metabolic
radiotracer, as opposed to a perfusion tracer,
potentially extends the time window for noninvasive
imaging of an ischemic event beyond the resolution
of symptoms. Targeting intracellular metabolic
processes could expand our ability to diagnose and
treat subclinical or progressive cardiovascular
disorders that often remain elusive with traditional
imaging approaches. These therapeutic strategies in
turn create a demand for accurate, sensitive, and
physiological evaluation of therapeutic effects.
The autonomic nervous system plays an
important role in many cardiac functions, including
cardiac rhythm, conduction, and repolarization.
Several specific neurotransmitters interact with
receptors on pre- and post-synaptic binding sites
regulating the complex system of the heart.
Abnormalities in this interaction result in a variety
of cardioneuropathies.
Receptor imaging can be helpful for
prognosticating patients with heart failure, diabetes,
ischemic heart disease, heart transplantation, drug-
induced cardiotoxicity, and dysautonomias.
Table 1: Examples of targets for molecular imaging of
different cardiac pathologic events.
Pathological/Biological
Process
Cellular/Molecular Targets
Ischemia / myocardial
damage
Renin-angiotensin system
Chemokine receptor
VEGFR receptor
Metabolic imaging
Fatty acids metabolism
Glucose metabolism
Cardiac neuronal imaging
Pre-/Post-synaptic – sympathic
and parasympathic innervation
Receptors / Channels
Acute myocardial infarct -
Acute Necrosis
Disrupted myocytes
Calcium rich-areas
Myosin
Atherosclerosis - vulnerable
plaques
Apoptosis
Inflammation
Adhesion
Lipoproteins
Angiogenesis
Vascular Pathology. The diagnosis of vulnerable
plaques remains an elusive goal in clinical medicine.
The most widely accepted features of vulnerable
plaques, such as large lipid core, increased
inflammatory milieu and thin fibrous caps, have
been well characterized through pathological
studies. The ability to image a vulnerable plaque in
susceptible patients should theoretically result in
useful prognostic information that can be used to
either monitor or treat patients at risk more
aggressively.
The relatively poor correlation between risk of
plaque rupture and the degree of luminal obstruction
exposes the crucial need for in vivo detection of the
processes underlying progressive plaque
destabilization.
In addition to the morphologic characteristics,
apoptosis and inflammation are two other important
indicators of plaque instability. Apoptotic
macrophage death results in enlargement of the
plaque necrotic core and positive vascular
remodeling, whereas apoptosis of the smooth muscle
cells leads to attenuation of the fibrous cap.
Finally, angiogenesis is defined as the formation
of new capillaries by cellular outgrowth from
existing microvessels. It plays a crucial role in the
response to ischemia that is associated with
PerfusionandSpecificRadioprobesforCardiacImaging
81
peripheral arterial disease and myocardial infarction.
Imaging angiogenesis would therefore be valuable in
assessing risk stratification of patients with arterial
occlusive disease.
Selected Examples of Molecular Imaging Probes.
The biodistribution of molecular imaging probes is
determined by specific interactions between the
radioactive molecule and its target, which can be for
example antigen, enzymatic or receptor-binding.
Therefore the probe should present a high affinity to
its target, and also a high specificity, resulting in its
selective uptake and distribution at the target tissues.
Different types of biomolecules and
radionuclides, both metallic and non-metallic, have
been explored in nuclear imaging.
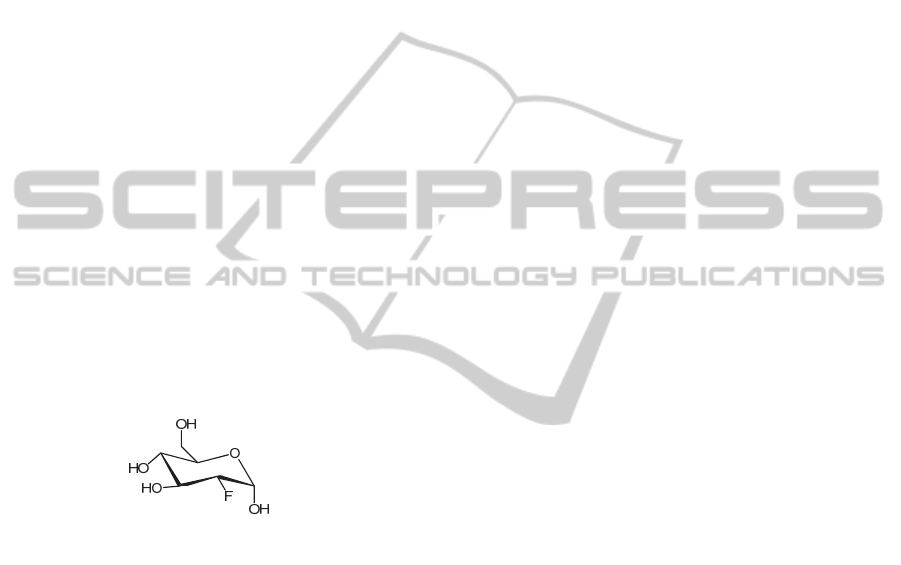
Within the field of cardiac molecular imaging
fluorine-18 fluorodeoxyglucose (
18
F-FDG) is the
most widely used agent (Figure 4).
18
F-FDG is a radiolabeled glucose analogue
transported into metabolically active cells, and
therefore it is an ideal agent for the assessment of
viable myocardium.
Moreover,
18
F-FDG presence is also correlated
with plaque macrophage content, and, therefore,
could be used as a surrogate reporter of this critical
cell involved in atherogenesis and plaque rupture
(Osborn, Jaffer, 2012).
Figure 4: Structure of
18
F-FDG.
The renin-angiotensin system (RAS) plays an
important role in regulating blood volume, arterial
pressure, cardiac and vascular function, and may
contribute to the pathogenesis of atherosclerosis.
The renin-angiotensin system is frequently activated
early in heart failure and is linked to left ventricular
remodeling and myocardial fibrosis.
A comprehensive in vivo approach to the study
of the RAS and its many components has been made
difficult by the complexity of the system. However,
this system has, at the same time, provided a number
of targets for nuclear imaging via radiolabeled
ligands, with special emphasis on the angiotensin-
converting enzyme (ACE).
The initial attempts at developing specific ACE-
binding radiotracers were made by use of
18
F–
labeled captopril, the first clinically available ACE
inhibitor. In normal rats, in vivo biodistribution at 30
minutes after injection revealed high uptake values
in the lungs, kidneys, and aorta, organs with known
high concentrations of ACE. This agent, however,
had a number of shortcomings that reduced its
potential as a suitable tracer for examining ACE
distribution, as it is believed to have a higher affinity
for vascular ACE than for tissue ACE and, thus, to
be less suited for examination of tissue-bound ACE
activity.
Another
18
F-labeled ACE inhibitor, lisinopril,
showed higher affinity for tissue ACE and allowed
higher resolution during in vitro autoradiography
when compared with
18
F-labeled captopril.
99m
Tc-
labeled lisinopril derivatives have been also
developed (Femia, et al., 2008) and recently it has
been shown that
99m
Tc-lisinopril localizes in the
heart of transgenic rats that over-express human
ACE-1 (Dilsizian, et al., 2012). The combination of
these studies has shown the feasibility of in vivo
imaging of ACE both by PET(
18
F) and SPECT
(
99m
Tc).
ACKNOWLEDGEMENTS
Fundação para a Ciência e a Tecnologia is
acknowledged for the Ciência 2007 grant to F
Mendes. The financial support of Covidien, Petten,
The Netherlands to IST/ITN is acknowledged.
REFERENCES
Notghi A., Low C. S., (2011) Myocardial perfusion
scintigraphy: past, present and future. Brit. J. Radiol.
84, S229–S236.
Germano G., Chua T., Kiat H., Areeda J. S., Berman D.
S., (1994) A quantitative phantom analysis of artifacts
due to hepatic activity in technetium-99m myocardial
perfusion SPECT studies. J. Nucl.Med. 35: 356-359.
Kailasnath P., Sinusas A. J., (2001) Technetium-99m-
labeled myocardial perfusion agents: Are they better
than thallium-201? Cardiol. Rev. 9: 160-172.
Kapur A., Latus K. A., Davies G., Dhawan R. T., Eastick
S., Jarritt P. H., Roussakis G., Young M. C.,
Anagnostopoulos C., Bomanji J., Costa D. C., Pennell
D. J., Prvulovich E. M., Ell P. J., Underwood S. R.
(2002) A comparison of three radionuclide myocardial
perfusion tracers in clinical practice: the ROBUST
study. Eur. J. Nucl. Med. Mol. Imaging. 29: 1608-
1616.
Llaurado J. G., (2001) The quest for the perfect
myocardial perfusion indicator...still a long way to go.
J. Nucl. Med. 42: 282-284.
Parker J. A., (2001) Cardiac nuclear medicine in
monitoring patients with coronary heart disease.
Semin. Nucl. Med. 31: 223-237.
CARDIOTECHNIX2013-InternationalCongressonCardiovascularTechnologies
82

Hatada K., Riou L. M., Ruiz M., Yamamichi Y., Duatti
A., Lima R. L., Goode A. R., Watson D. D., Beller G.
A., Glover D. K., (2004) 99mTc-N-DBODC5, a new
myocardial perfusion imaging agent with rapid liver
clearance: comparison with 99mTc-sestamibi and
99mTc-tetrofosmin in rats. J. Nucl. Med. 45: 2095-
2101.
Kim Y. S., Wang J., Broisat A., Glover D. K., Liu S.
(2008) Tc-99m-N-MPO: novel cationic Tc-99m
radiotracer for myocardial perfusion imaging. J. Nucl.
Cardiol. 15: 535-546.
Liu S., (2007) Ether and crown ether-containing cationic
99mTc complexes useful as radiopharmaceuticals for
heart imaging. Dalton Trans.: 1183-1193.
Liu Z., Chen L., Liu S., Barber C., Stevenson G., Furenlid
L., Barrett H., Woolfenden J., (2010) Kinetic
characterization of a novel cationic 99mTc(I)-
tricarbonyl complex, 99mTc-15C5-PNP, for
myocardial perfusion imaging. Journal of Nuclear
Cardiology 17: 858-867.
Garcia R. P., A.; Santos, I., (2009) Rhenium and
technetium complexes with anionic or neutral
scorpionates: An overview of their relevance in
biomedical applications. Inorg. Chim. Acta 362: 4315-
4327.
Santos I. P. P., Correia, J. D. G., (2005) Rhenium and
Technetium Complexes Anchored by Phosphines and
Scorpionates for Radiopharmaceutical Applications
In: W K (ed) Contrast Agents III -
Radiopharmaceuticals - From Diagnostics to
Therapeutics. Springer, Berlin, p 45.
Maria L., Cunha S., Videira M., Gano L., Paulo A., Santos
I. C., Santos I., (2007) Rhenium and technetium
tricarbonyl complexes anchored by pyrazole-based
tripods: novel lead structures for the design of
myocardial imaging agents. Dalton Trans.: 3010-
3019.
Maria L., Fernandes C., Garcia R., Gano L., Paulo A.,
Santos I. C., Santos I., (2009) Tris(pyrazolyl)methane
99mtc tricarbonyl complexes for myocardial imaging.
Dalton Trans.: 603-606.
Goethals L. R., Santos I., Caveliers V., Paulo A., De
Geeter F., Gano L., Fernandes C., Lahoutte T., (2010)
Rapid Hepatic Clearance of 99mTc-TMEOP: a New
Candidate for Myocardial Perfusion Imaging.
Contrast. Media Mol. Imaging.
Mendes F., Gano L., Fernandes C., Paulo A., Santos I.,
(2012) Studies of the myocardial uptake and excretion
mechanisms of a novel 99mTc heart perfusion agent
Nucl Med Biol, 39:207–213.
Mendes F., Paulo A., Santos I. (2011) Metalloprobes for
functional monitoring of tumour multidrug resistance
by nuclear imaging. Dalton Trans.,40:5377-539.
Osborn E. A., Jaffer F. A. (2012) The Year in Molecular
Imaging. JACC: Cardiovasc. Imag., 5, 317 – 328.
Femia F. J., Maresca K. P., Hillier S. M., Zimmerman C.
N., Joyal J. L., Barrett J. A., Aras O., Dilsizian,
Eckelman, Babich J. W., (2008) Synthesis and
Evaluation of a Series of 99mTc(CO)3+ Lisinopril
Complexes for In Vivo Imaging of Angiotensin-
Converting Enzyme Expression. J Nucl Med; 49,970–
977.
Dilsizian V., Zynda T. K., Petrov A., Ohshima S. et al.
(2012) Molecular Imaging of Human ACE-1
Expression in Transgenic Rats, JACC: Cardiovascular
Imaging, 5, 409-418.
PerfusionandSpecificRadioprobesforCardiacImaging
83
