
Experimental Study and Evaluation of Paper-based Inkjet Electrodes
for ECG Signal Acquisition
Ana Priscila Alves
1
, Jo
˜
ao Martins
2
, Hugo Pl
´
acido da Silva
1
, Andr
´
e Lourenc¸o
1,3
, Ana Fred
1
and Hugo Ferreira
2
1
Instituto de Telecomunicac¸
˜
oes, Instituto Superior T
´
ecnico, Avenida Rovisco Pais, 1, 1049-001 Lisboa, Portugal
2
Instituto de Biof
´
ısica e Engenharia Biom
´
edica, Faculdade de Ci
ˆ
encias da Universidade de Lisboa,
Alameda da Universidade, 1649-004 Lisbon, Portugal
3
Instituto Superior de Engenharia de Lisboa, Rua Conselheiro Em
´
ıdio Navarro, 1, 1959-007 Lisboa, Portugal
Keywords:
Electrodes, Paper, Inkjet, Electrocardiography, Device.
Abstract:
Applications involving biosignals, such as Electrocardiography (ECG), are becoming more pervasive with
the extension towards non-intrusive scenarios helping targeting ambulatory healthcare monitoring, emotion
assessment, among many others. In this study we introduce a new type of silver/silver chloride (Ag/AgCl)
electrodes based on a paper substrate and produced using an inkjet printing technique. This type of electrodes
can increase the potential applications of biosignal acquisition technologies for everyday life use, given that
there are several advantages, such as cost reduction and easier recycling, resultant from the approach explored
in our work. We performed a comparison study to assess the quality of this new electrode type, in which
ECG data was collected with three types of Ag/AgCl electrodes: i) gelled; ii) dry iii) paper-based inkjet
printed. We also compared the performance of each electrode when acquired using a professional-grade gold
standard device, and a low cost platform. Experimental results showed that data acquired using our proposed
inkjet printed electrode is highly correlated with data obtained through conventional electrodes. Moreover, the
electrodes are robust to high-end and low-end data acquisition devices.
1 INTRODUCTION
Pervasive healthcare applications are becoming an in-
valuable tool for regular and non-intrusive monitor-
ing. Biosignals play an important role in this kind
of applications since they give information about the
state of several vital organic tissues. Electrocardio-
graphic (ECG) signals are probably the most well-
known biosignals, and can be found in multiple ap-
plications in the medical and quality of life domains.
It is commonly used to assess the overall cardiac func-
tion, measure the rate and regularity of heartbeats,
and detect the presence of any pathology in the heart.
The classical acquisition methods used in clinical or
research studies typically recur to gelled silver/silver
chloride (Ag/AgCl) electrodes. Given that ECG data
acquisition has become more pervasive and inexpen-
sive, enabling an easy access to continuous monitor-
ing of the cardiac function, new and cheapest solu-
tions have been proposed, with more practical elec-
trodes and acquisition setups (Silva et al., 2011; Silva
et al., 2013).
Paper has several advantages for ECG data acqui-
sition in daily life scenarios; it enables: a) lower pro-
duction costs; b) easier recycling; and c) simpler pro-
duction, especially when considering the possibility
of inkjet printing. When compared to plastic sub-
strates such as polyethylene terephtalate (PET, ≈ 2
cent dm
−2
) and polymide (PI, ≈ 30 cent dm
−2
), pa-
per has significantly lower production costs (≈ 0.1
cent dm
−2
). In addition to this, considering the active
disassembly design principles (Chiodo and Ijomah,
2012), paper is a good choice due to its environmen-
tally friendly characteristics. Recently, it has been
considered as a potential substrate for low-cost flex-
ible electronics (Siegel et al., 2010; Leenen et al.,
2009), which motivated us to do research on the pos-
sibility of using paper-based electrodes for biosignals
acquisition. With such an approach and its ready
availability, the electrodes can even be produced by
the user himself or his caregivers.
The deposition of the conductive part of the elec-
trodes to the paper substrate can be made recurring
to photo-lithography, vacuum processes or printing
275
Alves A., Martins J., Plácido da Silva H., Lourenço A., Fred A. and Ferreira H..
Experimental Study and Evaluation of Paper-based Inkjet Electrodes for ECG Signal Acquisition.
DOI: 10.5220/0004720802750281
In Proceedings of the International Conference on Physiological Computing Systems (PhyCS-2014), pages 275-281
ISBN: 978-989-758-006-2
Copyright
c
2014 SCITEPRESS (Science and Technology Publications, Lda.)

techniques. The use of printing techniques for fab-
ricating electronics has several advantages over labo-
ratory scale and subtractive batch processes (Tobj
¨
ork
and
¨
Osterbacka, 2011); printing is fast, low-cost, and
widely used. In particular digital inkjet printing,
which has been used as a research tool, is facilitating
initial explorations of various aspects of printed elec-
tronics targeting the consumer market (Singh et al.,
2010). The focus of this work was to explore the po-
tential use of paper-based inkjet printed electrodes for
ECG signal acquisition.
The most commonly used type of electrode is the
gelled Ag/AgCl electrode; however, to make an ac-
quisition setup more convenient for everyday use ap-
plications, other alternatives are emerging. Previous
work from our group has started to explore the use
of dry Ag/AgCl electrodes (Silva et al., 2011), which
usually leads to signals with lower signal-to-noise ra-
tio, although still suitable for monitoring or other non-
intrusive applications. Thus, to study the characteris-
tics of the paper-based inkjet printed electrodes, we
perform a comparative study against the most com-
mon alternatives: i) gelled; ii) dry.
The remainder of the paper is organized as fol-
lows: in Section 2 we describe the proposed elec-
trodes, focusing on their production and main char-
acteristics; Sections 3 and 4 present the methodology
applied in the comparison of the different electrode
types and their quantitative evaluation; and finally, in
Sections 5 and 6 we provide a summary of the exper-
imental results and outline the main conclusions.
2 PAPER-BASED INKJET
PRINTED ELECTRODES
The possibility of printing materials using inkjet tech-
nology brought several advantages to the conven-
tional manufacturing procedures used, such as photo-
lithography, transfer printing, among others. Compar-
ing with those standard techniques for patterning thin
films with high precision, some differences stand out.
The appeal of inkjet technology lies in the fact that
it is based on contactless deposition, which implies a
lesser risk of contaminating the material, it is a mask-
less approach that makes an intuitive procedure, and
it is an additive procedure, i.e., it is possible to print
over a previous printed pattern (Singh et al., 2010).
Producing electrodes by inkjet printing enables
the use of thin and flexible substrates that may also be
biocompatible, examples of which are polydimethyl-
siloxane (PDMS) or biocellulose. On the other hand,
low-cost paper-like substrates such as photo paper can
be used as an alternative substrate and several conduc-
tive inks can already be used, such as silver, gold or
conductive polymer (Calvert, 2001)).
We fabricated the electrodes using photo paper as
substrate, due to its flexibility, availability, reduced
thickness (230 µm) and easy maneuverability. To
create the conductive part of the electrode we used
a commercial printable silver ink from SunTronic,
which is composed of silver nanoparticles and has
been shown to provide good electrical conductivity
for electronic applications.
The electrodes devised in the scope of our work
were designed as a flat rectangle shape, with dimen-
sions of 8 cm length, 3 cm width and approximately
1 µm thick. Each electrode has a total of 24 cm
2
of
area in contact with the skin. The electrodes were first
printed with four silver layers and aftwerwards sub-
jected to heat treatment during 20 minutes at a tem-
perature of 85
◦
C. With this heat treatment, we ob-
tained a silver resistivity of 1.68 ×10
−6
Ω.m .
The second step of the fabrication process was
to produce a layer which enables the transduction
of ionic concentrations measured by electrodes into
electrical potentials. At the skin-electrode interface,
the ionic signal (Cl
-
ion transports the charge) is trans-
formed into an electric signal. Likewise, in common
silver electrodes this layer is typically made of AgCl
(Clark et al., 2009). The formation of this layer was
achieved by adding Cl
-
ions, enabling a reaction be-
tween Ag and Cl to produce AgCl. However, due to
the thin layer of silver and the fragility of the photo
paper, the amount and the manner of introducing Cl
-
ions is important. This process was optimized by us-
ing commercial bleach deposited by an airbrush at a
distance of approximately 30 cm.
The third step in the production of these electrodes
was focused on ensuring a good, long lasting, and
practical contact between the electrodes and the ac-
quisition hardware. To facilitate the connection of ca-
bles and make the electrodes practical for regular use,
we use a metal stud and conductive snap. The snaps
were placed in the back of the printed surface and the
communication to the front was made through a hole
filled with a conductive silver paste from Agar Scien-
tific. We estimated that each electrode produced with
the procedure described would cost, approximately,
0.03e.
Figure 1: Electrode leads placement.
PhyCS2014-InternationalConferenceonPhysiologicalComputingSystems
276
3 METHODOLOGY
We benchmarked the performance of our paper-based
inkjet printed electrodes for ECG data acquisition,
comparing them both to standard pre-gelled Ag/AgCl
electrodes, and to the dry electrodes approach that we
have been recently following (Silva et al., 2011). Ref-
erence data was collected using a BIOPAC biosignal
acquisition unit, which has seen extensive use in the
research domain and is considered to be a gold stan-
dard in biomedical research. However, this system
has restricted operations and experimenting new cus-
tomized solutions can damage the device. As such,
we have used a BITalino acquisition system (Alves
et al., 2013; Guerreiro et al., 2013), which give us a
higher control over the system to try different experi-
mental setups.
This work is aligned with our research towards
off-the-person ECG sensing (Silva et al., 2013), rea-
son for which the ECG signals were acquired in the
palmar region of the left and right hands, as illus-
trated in Figure 1. The electrodes used for data acqui-
sition with the BIOPAC were always the pre-gelled
Ag/AgCl, while with the BITalino we tested the pre-
viously mentioned 3 types of electrodes.
We devised our comparative study in two objec-
tives:
1. comparison of the BITalino performance with a
gold standard acquisition system, the BIOPAC;
2. comparison of electrodes for ECG acquisition.
The BITalino acquisition device adopts the 2-
electrode approach with virtual ground, while the
BIOPAC system is designed to collect data with the
ground electrode. In order to inquire the BIOPAC
performance after removing the ground electrode, we
performed two experiments, with and without the
ground electrode. To evaluate the performance of the
dry, and paper-based inkjet electrodes in the ECG ac-
quisition, we did 2 experiments in which we com-
pared them with the pre-gelled ones. The experiments
are summarized in Table 1.
Table 1: Summary of the experiments.
Experiment
BIOPAC
BITalino
type GND
1 Gel Yes Gel
2 Gel No Gel
3 Gel No Dry
4 Gel No Paper
Each experiment consisted of a 30 seconds record-
ing performed simultaneously with the BIOPAC and
the BITalino; we used a sampling rate of 1000 Hz in
both devices and a 12-bit resolution for the BIOPAC,
whereas the BITalino has a 10-bit resolution. The
BIOPAC raw data was reduced to 10 bits, to be at
the same resolution as the BITalino signals. We
have collected raw ECG data from 20 subjects in a
static standing position, with the electrodes applied
as shown in Figure 1
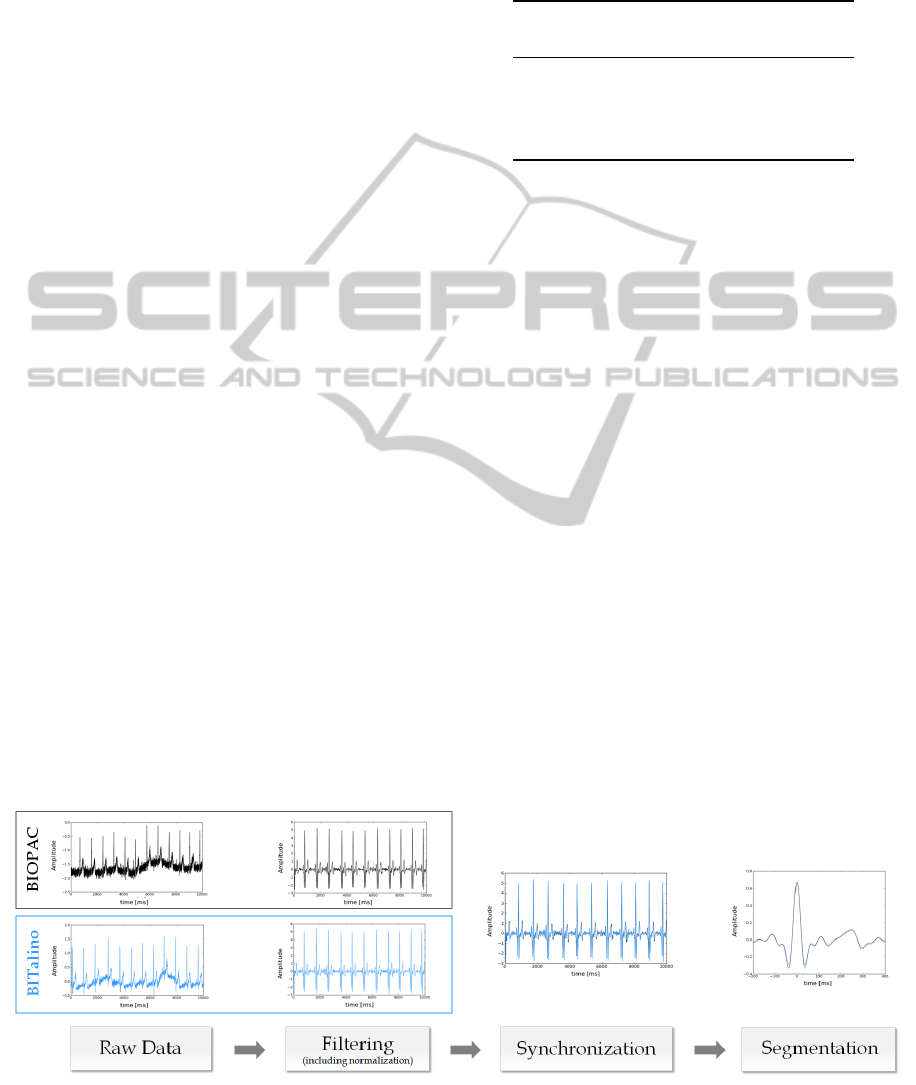
The data obtained by each device was pre-
processed in three main steps, as represented in Fig-
ure 2. Taking the raw data as input, the baseline
wander was corrected through a two-stage median
filter, as proposed by (De Chazal et al., 2004), and
the signals were filtered using a Finite Impulse Re-
sponse (FIR) bandpass filter with a Hamming window
of 300 ms, and cutoff frequencies of 5 − 20Hz. The
filtered signals were normalized to their maximum
and minimum amplitudes, where the original signal
is subtracted of its mean, and divided by its standard
deviation. To prevent any possible electrical interfer-
ence between the devices prone to bias the results and
resulting from a hard wired connection between both
devices, we chose to do the synchronization using the
Figure 2: Block diagram of the pre-processing steps we have performed, to compare the signals acquired from both devices.
The curves plotted in black were acquired using the BIOPAC while the blue ones with the BITalino.
ExperimentalStudyandEvaluationofPaper-basedInkjetElectrodesforECGSignalAcquisition
277

RR time intervals. Given that the comparison of the
ECG data obtained from two independent systems can
only be correctly performed for data expressed in the
same time base, our synchronization method consists
on the following steps:
1. Detection of the QRS complex in each indepen-
dent signal, using the method proposed by (En-
gelse and Zeelenberg, 1979)
2. Let RR
BIOPAC
= {RR
BIOPAC
0
, ..., RR
BIOPAC
n
} and
RR
BITalino
= {RR
BITalino
0
, ..., RR
BITalino
m
} be a set
of RR time intervals for the n and m heartbeat
waveforms detected respectively in the BIOPAC
and BITalino ECG time series.
3. Construct a matching matrix, M, in which the en-
try M(i, j) corresponds to the absolute value of
the difference between the RR time intervals ex-
tracted from the BIOPAC and BITalino ECG time
series, that is:
M(i, j) = |RR
BITalino
i
− RR
BIOPAC
j
| (1)
4. Let #M be the number of items where M(i, j) ≤
RR
th
5. If #M > Sync
th
, the synchronization is complete.
Otherwise, go to next step.
6. Consider RR
BITalino
(k) =
{RR
BITalino
k
, ..., RR
BITalino
m
}. Repeat steps
3 and 4 for each value of k ∈ {1, ..., m}
and compute each value of M(i, j) =
|RR
BITalino
i
(k) − RR
BIOPAC
j
|.
7. Find the k value where #M is higher
8. Synchronize the signals by applying a delay of k
samples to the BITalino signal.
The acquisition was always initiated first with the
BITalino, so it has the higher time series. We defined
2 thresholds in the synchronization method, Sync
th
and RR
th
. The Sync
th
value applied was 20, since it is
approximately the minimum number of heartbeats ex-
pected in a 30 seconds ECG signal. The RR
th
thresh-
old represents the minimum difference of RR time in-
tervals, from different acquisitions, where the R peaks
are considered to match in the time domain. Since
the acquisitions were performed by two different sys-
tems, it is expected a small deviation between the in-
stants where the same R peaks occur. Therefore, we
considered that 5 ms is the maximum value where the
R peaks are considered to occur in the same instant.
Finally, the individual heartbeat waveforms were seg-
mented and scaled between 0 and 1; we consider the
heartbeat waveform to be the [−200; 400]ms interval
around the R peak instant.
4 EVALUATION METRICS
Two metrics were employed for numerical evaluation
purposes, namely the Signal-to-Noise Ratio (SNR)
computed from the data collected with both devices
for each of the 4 experiments, and the Root Mean
Square Error (RMSE) of the cosine distance, to as-
sess the morphological correlation between the heart-
beat waveforms obtained with the BIOPAC and the
BITalino, when using each type of electrodes. For
the SNR calculation, we considered the interest sig-
nal to be concentrated on the 5 − 20 Hz band of its
frequency spectrum, and the remainder as noise. For
each record we calculated the difference between the
SNR obtained from BITalino and BIOPAC acquisi-
tion.
Figure 3 illustrates an example of the frequency
spectrum of ECG data acquired in both devices, for
(a) BIOPAC (b) BITalino
Figure 3: Example of the ECG signal frequency spectrum for data collected with each acquisition device in one of the
recording sessions. The blue region shows the interest spectral band and the remainder the noise.
PhyCS2014-InternationalConferenceonPhysiologicalComputingSystems
278
one of the test subjects in the experiment 1. The 50
Hz power line interference is visible in both signals;
however, since the BITalino ECG sensor has an ana-
log band pass filter from 0.5 to 40Hz, the higher fre-
quencies are almost eliminated, contrary to what hap-
pens with the BIOPAC.
For the cosine distance calculation, the synchro-
nized signals were segmented into individual heart-
beat waveforms, and the distance between a given
segment in the BIOPAC time series and the matching
segment in the BITalino time series was calculated.
The cosine distance, D
cos
, between the signals x and
y is given by Equation 2
D
cos
(x, y) = 1 −
∑
m
k=1
x[k]y[k]
p
∑
m
k=1
x[k]
2
∑
m
k=1
y[k]
2
, (2)
The reason why we have calculated the cosine dis-
tance for each heartbeat, instead of using the entire
signal, is due to the fact that we were only interested
in the ECG waveform shape, which is comprised in
the heartbeat region. To validate the similarity be-
tween the signals acquired from the two devices, we
compute the RMSE, as defined in Equation 3
RMSE(x, y) =
s
∑
N
j=1
D
cos
j
(x, y)
2
N
(3)
5 EXPERIMENTAL RESULTS
The results obtained for each experiment in the 20
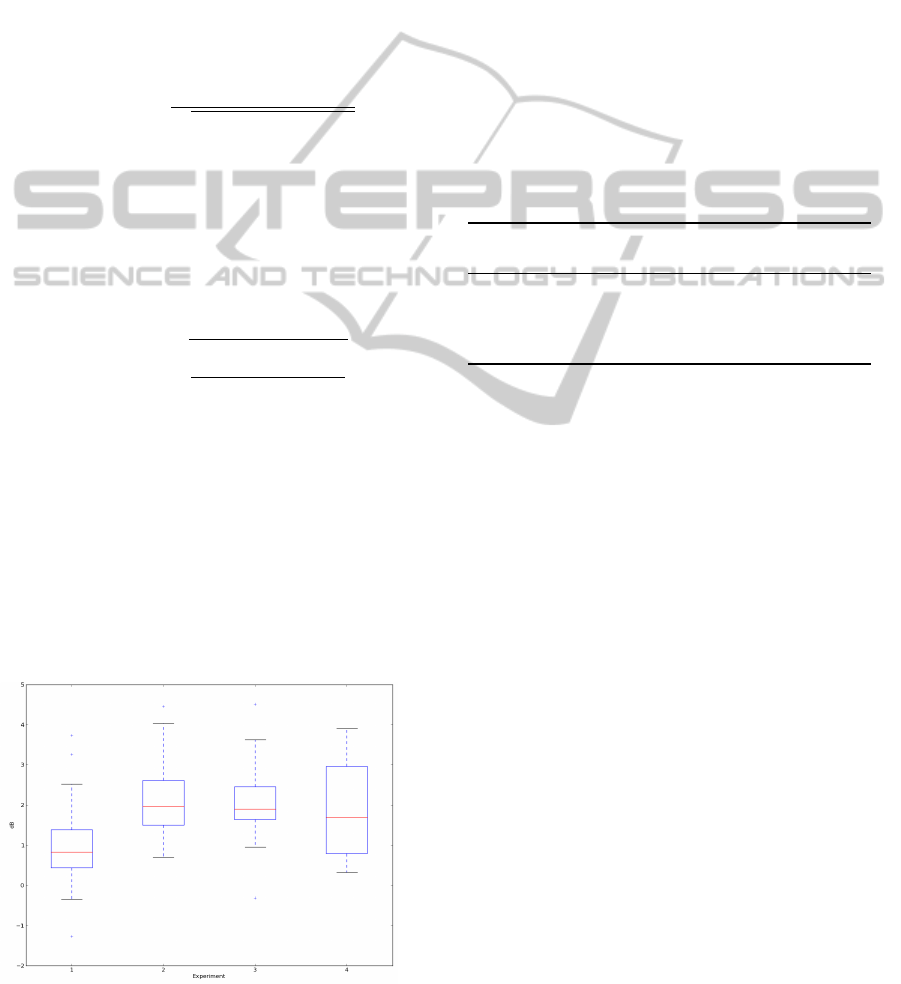
subjects are represented in Figure 4.
The box plots display the distribution of the differ-
ence between BITalino and BIOPAC SNR, for each
experiment, across all the subjects. The height of
the box plot indicates the degree of dispersion, the
band inside the box represents the median, and the
Figure 4: Boxplot of the difference between BITalino and
BIOPAC Signal-to-Noise Ratio for each experiment.
bottom and top of the box are the first and third quar-
tiles. The smallest SNR difference between devices
was obtained in the experiment 1, where the median
value is lower and the degree of dispersion is reduced.
This was already expected since the presence of the
ground electrode in the BIOPAC device and the use
of gelled electrodes in both systems correspond to the
best case scenario in which the amount of captured
noise is minimal. The higher dispersion obtained was
in the experiment 4, due to higher noise presence in
the signals.
Table 2 summarizes the results obtained for the
signals collected using each device. In all the experi-
ments, the SNR of BITalino was higher than BIOPAC,
which was already expected due to the analogic filter-
ing occuring in the BITalino ECG sensor.
Table 2: Experimental Results from BIOPAC and BITalino
ECG signals acquisition, in the 4 experiments.
Experiment RMSE
SNR [dB] SNR [dB]
BITalino BIOPAC
1 0.0043 ± 0.0053 −1.02 ± 2.04 −2.04 ± 2.31
2 0.0042 ± 0.0039 −1.19 ± 1.84 −3.35 ± 2.45
3 0.0063 ± 0.0055 −1.62 ± 2.21 −3.69 ± 2.54
4 0.0042 ± 0.0043 −1.87 ± 2.14 −3.80 ± 2.66
The lowest value of SNR with the BITalino de-
vice was obtained in the experiment 4, when using
the paper electrodes, indicating a higher noise pres-
ence. In what concerns the morphological correla-
tion between waveforms, all the experiments have
shown a high similarity between the ECG signals ob-
tained from both devices. The signals acquired have
a good approximation to the well known prototypi-
cal ECG waveform, providing an easy identification
of the characteristic P-QRS-T complexes. Figure 5
presents an overlay with all the individual heartbeat
waveforms collected in one of the recording sessions,
showing the median and standard deviation of all the
segments obtained from both devices in the four ex-
periments. As we can see, the waveform morphology
is maintained throughout the experiments and is virtu-
ally indistinguishable between devices and materials.
From the cosine distance results, we have calcu-
lated the Root-Mean-Square Error (RMSE), and the
results are described in Table 2. For all the experi-
ments, we verified very low RMSE values, indicat-
ing that the signals obtained from all three types of
electrodes retain much of the waveform morphology
when compared to the signals obtained with the gold
standard BIOPAC setup. An interesting finding is
that the inkjet printed electrodes shows a very good
performance when compared to the other electrodes,
with a RMSE of 0.0042, while with the dry elec-
ExperimentalStudyandEvaluationofPaper-basedInkjetElectrodesforECGSignalAcquisition
279
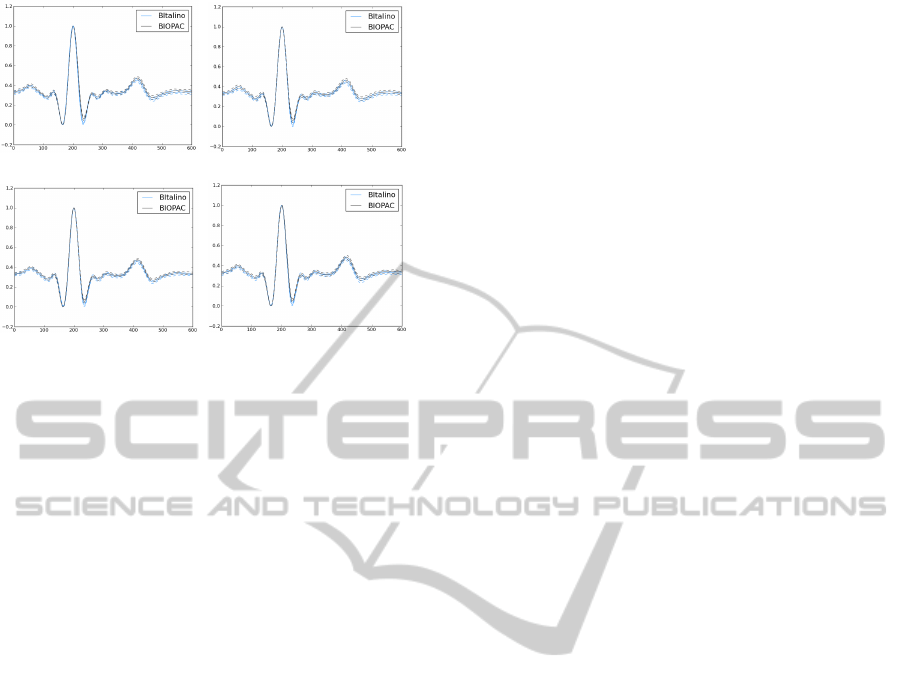
(a) Exp 1 (b) Exp 2
(c) Exp 3 (d) Exp 4
Figure 5: Segmented heartbeat waveforms from the BITal-
ino (blue) and the BIOPAC (grey); the solid wave represents
the mean, and dashed line the standard deviation.
trodes we obtained the worst results, with a RMSE of
0.0063. Although the signals obtained with the paper-
based electrodes present a lower signal-to-noise ra-
tio, the ECG morphology is maintained, which results
in a similar performance to that found for the case
in which standard clinical-grade pre-gelled Ag/AgCl
electrodes are used. Moreover, the signals acquired
with the BITalino device are highly correlated to those
obtained with the BIOPAC, actually exhibiting lower
noise levels in raw ECG signals.
6 CONCLUSIONS
In this paper we have proposed and evaluated paper-
based inkjet printed electrodes for ECG data acquisi-
tion. We presented the fabrication steps, and bench-
marked our electrodes against standard clinical-grade
pre-gelled Ag/AgCl electrodes, and dry electrodes.
Data acquisition was performed using a BIOPAC sys-
tem, considered to be a gold standard within the
biosignal research community, although due to the
fact that it is a closed system, we have also supported
our analysis on the BITalino, a physiological comput-
ing platform first introduced by our team.
The collected data was evaluated using the Signal-
to-Noise Ratio (SNR), and a morphological wave-
form correlation index based on the Root Mean
Square Error (RMSE). Experimental results have
shown that the proposed approach explored in this
work achieves comparable performance when com-
pared with a reference sensor. Our evaluation
has revealed that the heartbeat waveforms measured
through the proposed approach are nearly identical to
those obtained with the gold standard equipment.
This approach opens new possibilities in the field
of biosignals, enabling people (e.g. patients and/or
caregivers) to have easier access to consumables in
continuous ambulatory monitoring scenarios. We be-
lieve our approach to have the threefold advantage
of reducing production costs, being easier to recycle,
and being more accessible when compared to conven-
tional approaches.
ACKNOWLEDGEMENTS
This work was partially funded by Fundac¸
˜
ao para
a Ci
ˆ
encia e Tecnologia (FCT) under the grants
PTDC/EEI-SII/2312/2012, SFRH/BD/65248/2009
and SFRH/PROTEC/49512/2009, whose support the
authors gratefully acknowledge.
REFERENCES
Alves, A. P., Silva, H., Lourenc¸o, A., and Fred, A. (2013).
BITalino: A Biosignal Acquisition System based on
Arduino. In Proceeding of the 6th Conference on
Biomedical Electronics and Devices (BIODEVICES).
Calvert, P. (2001). Inkjet Printing for Materials and De-
vices. Chemistry of Materials, 13(10):3299–3305.
Chiodo, J. and Ijomah, W. (2012). Use of active disassem-
bly technology to improve remanufacturing produc-
tivity: automotive application. International Journal
of Computer Integrated Manufacturing, 0(0):1–11.
Clark, J. W., Neuman, M. R., Olson, W. H., Peura, R. A.,
and Primiano, F. P. (2009). Medical instrumentation:
application and design. John Wiley & Sons, inc,
fourth edition.
De Chazal, P., O’Dwyer, M., and Reilly, R. (2004). Auto-
matic classification of heartbeats using ECG morphol-
ogy and heartbeat interval features. IEEE Transac-
tions on Biomedical Engineering, 51(7):1196–1206.
Engelse, W. A. H. and Zeelenberg, C. (1979). A single scan
algorithm for QRS-detection and feature extraction.
Computers in Cardiology, 6:37–42.
Guerreiro, J., Silva, H., Lourenc¸o, A., Martins, R., and Fred,
A. (2013). BITalino:A Multimodal Platform for Phys-
iological Computing. In Proceeding of the 10th In-
ternational Conference on Informatics in Control, Au-
tomation and Robotics (ICINCO).
Leenen, M. A. M., Arning, V., Thiem, H., Steiger, J.,
and Anselmann, R. (2009). Printable electronics:
flexibility for the future. physica status solidi (a),
206(4):588–597.
Siegel, A. C., Phillips, S. T., Dickey, M. D., Lu, N., Suo, Z.,
and Whitesides, G. M. (2010). Foldable printed cir-
cuit boards on paper substrates. Advanced Functional
Materials, 20(1):28–35.
PhyCS2014-InternationalConferenceonPhysiologicalComputingSystems
280

Silva, H., Carreiras, C., Lourenc¸o, A., and Fred, A. L. N.
(2013). Off-the-person electrocardiography. In In-
ternational Congress on Cardiovascular Technologies
(CARDIOTECHNIX).
Silva, H., Lourenc¸o, A., Lourenc¸o, R., Leite, P., Coutinho,
D., and Fred, A. (2011). Study and evaluation of a sin-
gle differential sensor design based on electro-textile
electrodes for ECG biometrics applications. In Pro-
ceedings of the IEEE Sensors Conference, pages 1764
– 1767.
Singh, M., Haverinen, H. M., Dhagat, P., and Jabbour, G. E.
(2010). Inkjet printing-process and its applications.
Advanced Materials, 22(6):673–685.
Tobj
¨
ork, D. and
¨
Osterbacka, R. (2011). Paper electronics.
Advanced Materials, 23(17):1935–1961.
ExperimentalStudyandEvaluationofPaper-basedInkjetElectrodesforECGSignalAcquisition
281
