
A Novel Pipeline for Identification and Prioritization of Gene Fusions in
Patient-derived Xenografts of Metastatic Colorectal Cancer
Paciello Giulia
1
, Andrea Acquaviva
1
, Consalvo Petti
2
, Claudio Isella
2
, Enzo Medico
2,3
and Elisa Ficarra
1
1
Department of Control and Computer Engineering, Politecnico di Torino, Corso Duca degli Abruzzi, Turin, Italy
2
Laboratory of Oncogenomics, Institute for Cancer Research at Candiolo (IRCC),
Strada Provinciale 142, Candiolo, Turin, Italy
3
Department of Oncology, Universita’ di Torino, Candiolo, Turin, Italy
Keywords:
RNA-sequencing, Metastatic Colorectal Cancer, Patient-derived Mouse Xenografts, Artifacts.
Abstract:
Metastatic spread to the liver is a frequent complication of colorectal cancer (CRC), occurring in almost
half of the cases, for which personalized treatment strategies are highly desirable. To this aim, it has been
proven that patient-derived mouse xenografts (PDX) of liver-metastatic CRC can be used to discover new
therapeutic targets and determinants of drug resistance. To identify gene fusions in RNA-Seq data obtained
from such PDX samples, we propose a novel pipeline that tackles the following issues: (i) discriminating
human from murine RNA, to filter out transcripts contributed by the mouse stroma that supports the PDX;
(ii) increasing sensitivity in case of suboptimal RNA-Seq coverage; (iii) prioritizing the detected chimeric
transcripts by molecular features of the fusion and by functional relevance of the involved genes; (iv) providing
appropriate sequence information for subsequent validation of the identified fusions. The pipeline, built on top
of Chimerascan(R.Iyer, 2011) and deFuse(McPherson, 2011) aligner tools, was successfully applied to RNA-
Seq data from 11 PDX samples. Among the 299 fusion genes identified by the aforementioned softwares,
five were selected since passed all the filtering stages implemented into the proposed pipeline resulting as
biologically relevant fusions. Three of them were experimentally confirmed.
1 INTRODUCTION
It is currently known that cancer derives from per-
manent alterations of the cellular DNA, leading to
aberrant growth, invasion of adjacent tissues and
metastatic diffusion at distant sites(Hanahan, 2000).
Among the various DNA alterations, chromosomal
rearrangements leading to gene fusions play a cen-
tral role in the initial steps of many pathologies
such as leukaemias, sarcomas and common epithelial
neoplasms, like breast, colorectal and prostate can-
cer(Aman, 1999). The impact of gene fusions on cel-
lular behavior is due to functional alteration of one
or both the genes involved in the chromosomal re-
arrangements that give rise to chimeric transcripts.
Typical consequences of gene fusions, at the RNA
and protein level, are strong variation of expression,
removal of regulatory domains, forced oligomeriza-
tion, change of the subcellular location or acquisi-
tion of novel binding domains(Edwards, 2010). Gene
fusions can therefore have important prognostic and
therapeutic implications in the management of malig-
nancies, as shown in recent studies(Mitelman, 2007).
Data produced by Next Generation Sequenc-
ing (NGS) technologies are nowadays considered
very useful in order to detect genetic abnormali-
ties(Ansorge, 2009)(Mardis, 2008)(Metzker, 2010).
In particular, concerning chimeric transcripts iden-
tification, the analysis of the so called paired-end
RNA-Seq reads can be considered a powerful strat-
egy as shown by Maher and colleagues(Maher, 2009)
and confirmed by the subsequent development of nu-
merous tools to perform such activity(McPherson,
2011)(R.Iyer, 2011)(Abate, 2012). Paired-end reads,
differently from single-end reads, are obtained by se-
quencing nucleic acid fragments at both the 5’ and
3’ ends. When the two sequenced portions of the
fragment, called mates, align on different genes, it is
likely that the fragment is originated by a chimeric
transcript. We applied this strategy to identify rele-
vant gene fusions in colorectal cancer (CRC), one of
the most frequent cancers worldwide(Walther, 2000).
142
Giulia P., Acquaviva A., Petti C., Isella C., Medico E. and Ficarra E..
A Novel Pipeline for Identification and Prioritization of Gene Fusions in Patient-derived Xenografts of Metastatic Colorectal Cancer.
DOI: 10.5220/0004799401420148
In Proceedings of the International Conference on Bioinformatics Models, Methods and Algorithms (BIOINFORMATICS-2014), pages 142-148
ISBN: 978-989-758-012-3
Copyright
c
2014 SCITEPRESS (Science and Technology Publications, Lda.)

CRC is frequently complicated by liver metastasis,
and features a remarkable heterogeneity in terms of
molecular pathogenesis, natural history and response
to treatment(Siena, 2009)(Cunningham, 2010). A
recent advance in the characterization of such het-
erogeneity has been brought forward by the prop-
agation of human neoplastic tissue in immunodefi-
cient mice, the so-called patient-derived xenograft
(PDX) approach. As proven by Bertotti and col-
leagues(Bertotti, 2011), PDXs of human metastatic
colorectal cancer can be reliably exploited to discover
novel determinants of therapeutic response and new
oncoprotein targets. PDXs are indeed able to con-
serve the inter-individual diversity and the genetic
heterogeneity typical of the tumors of origin and at
the same time to reproduce the disease responses in
humans.
In the present work we searched for chimeric tran-
scripts in eleven PDXs of metastatic CRC, by ana-
lyzing Illumina RNA-Seq data consisting of 100-base
pair (bp) long paired-end reads. RNA was extracted
from PDXs at the second passage of propagation in
mice. By this stage human stromal cells, not capa-
ble of growing in the murine context, are replaced
by mouse stromal cells. As a consequence, the ex-
tracted RNA is a mixture of human RNA, originated
from neoplastic cells, and murine RNA from stromal
cells. This required a dedicated step in the RNA-Seq
analysis pipeline to distinguish reads originated from
RNAs of the two species. The experimental setup is
reported in Figure 1.
Figure 1: Experimental Setup. The activities highlighted in
blue boxes are respectively that implemented in the pipeline
objective of this work and the validation of the obtained re-
sults.
In particular rhe proposed research is inspired by
the observation that fusion detection tools usually
produce in the preliminary steps of their workflow a
conspicuous number of putative gene fusions not fea-
sible for in lab validation. To shrink down these can-
didates lists, gene fusions detection tools implement
inside their algorithms different kind of filters gener-
ally efficient in discarding false positive fusion tran-
scrpits but at the same time determining poor sensi-
tivity level of the detection. Therefore, as reported By
Abate and colleagues (Abate, 2012) and Carrara and
colleagues(Carrara, 2013) because the heterogeneity
of algorithms and filters applied, the identified fusions
usually poorly overlap with a high rate of false posi-
tives, but also of false negatives, leading to the need
of considering the union set of fusion genes detec-
tion tool outputs for in lab validation. The number
of fusions to be tested becomes in this way not feasi-
ble even considered that the biologically relevant fu-
sions in a sample are usually very few if any(Ozsolak,
2011).
With the aim of reducing the union set of candi-
dates detected by two fusion genes discovery tools (i.e
Chimerascan(R.Iyer, 2011) and defuse(McPherson,
2011)) in the aforementioned PDXs samples, we pro-
posed a novel pipeline characterized by the reimple-
mentation of some modules proper of fusion genes
detection tools and ad hoc scripts developed to per-
form different filtering stages.
At the days no tools or algorithms implementing
the analysis performed by our pipeline can be iden-
tified since all sequencing studies in PDXs such as
those of Rossello and colleagues, Conway and col-
leagues, Valder and colleagues(Rossello, 2013)(Con-
way, 2012)(Valdes, 2013) circumscribed at the dis-
crimination between murine and human reads don’t
relying on fusion genes detection beyond their priori-
tizazion.
The whole workflow is implemented in order to:
(i) take account for the murine stroma; (ii) consider
the contingent PCR artifacts; (iii) evaluate the role of
different kind of reads in the chimeric transcript reli-
ability to maximize sensitivity also at low sequencing
coverage; (iv) integrate biological and functional in-
formation about the gene fusions. Among the priori-
tized fusions, three have been at the moment experi-
mentally confirmed in lab with PCR.
2 METHODS
The proposed pipeline is characterized by different
activities and filtering stages as shown in Figure 2.
In the following all the steps will be detailed.
First Filtering Stage: Gene Fusions Annotation
and Selection.
The list of gene fusions detected using Chimeras-
can(R.Iyer, 2011) and defuse(McPherson, 2011) tools
with default run parameters constitute the input of
the first step of the proposed workflow on the eleven
RNA-Seq samples under examination. The choice of
Chimerascan(R.Iyer, 2011) and deFuse(McPherson,
ANovelPipelineforIdentificationandPrioritizationofGeneFusionsin
Patient-derivedXenograftsofMetastaticColorectalCancer
143

Figure 2: Pipeline workflow. Activities are represented in
yellow boxes whereas input and output in blue ones.
2011) tools is dictated by a recent research performed
by Carrara and colleagues(Carrara, 2013). Chimeras-
can(R.Iyer, 2011) and deFuse(McPherson, 2011)
tools were indeed proven to achieve good sensitivity
levels on real dataset even if generally provide a
remarkable number of false positive chimeras. This
negative feature has been however properly managed
thanks to the following different filtering stages. Fur-
thermore for what is concerning Chimerascan(R.Iyer,
2011) and defuse(McPherson, 2011) run parame-
ters they can be conveniently triggered according
to the specific requirements even if we evaluated
that from a computational point of view it is more
convenient to impose restrictive mapping policies
in the following phases of the proposed pipeline.
Chimeric transcripts output files, containing all the
fusions detected in the samples, are automatically
elaborated taking advantage of an annotation tool
(http://sourceforge.net/projects/pegasus-fus/) able
to perform on the detected fusions, protein domain
and functional analysis. The achieved information
provide a more detailed overview on each chimeric
transcript supplying knowledge about the presence
of kinases, the reading frame, the domain conserved
or loss, and the breakpoint regions. Of essential
importance in this phase are also the data provided
by the chimeric transcripts detection tools for what is
concerning the reads used to identify a gene fusion.
Chimerascan(R.Iyer, 2011) and deFuse(McPherson,
2011) indeed, in order to score the fusions detected
in the samples, provide the number of reads used
to define the fusion: They distinguish between
encompassing reads, in other word paired-end reads
with the two mates mapped respectively on the two
different partner genes of the chimeric transcript and
split mates, representing those mates that harbor the
gene fusion breakpoint. Starting from the information
collected using the aforementioned annotation tool,
the gene fusions detection tools outputs and thanks
to biological considerations about the function of the
genes involved in the fusion a first step of filtering
is performed. Only those fusions characterized by a
certain threshold number of split reads that will be
discussed in Results Section and satisfying also at
least another one criteria of those previously listed
will be considered for further evaluations.
Second Filtering Stage: Breakpoint Sequences
Analysis and Gene Fusions Selection.
Starting from the prioritized list of fusion gene
candidates a new stage of filtering is applied. The
fusion sequence of each chimeric transcript, provided
by defuse(McPherson, 2011) and deduced from
the split reads for what is concerning Chimeras-
can(R.Iyer, 2011) is here analysed. Main objective
of this filtering stage consists essentially in the
retrieving of those gene fusions sequences that could
account for the translation of the chimeric transcript
into a functional protein. The presence of a Kozac
sequence in the 5’ partner gene generally account
for an ATG triplet (starting site of the translation
process) downstream that allows the beginning of
the translation process. In this phase also chimeric
transcript sequences characterized by an in frame
configuration have been however selected for further
analyses since we considered the case in which the
ATG triplet is located upstream with respect to the
starting point of the sequencing.
Third Filtering Stage: Paired-end Reads Mapping
and Gene Fusions Selection.
For the gene fusions selected in the previous step
the mapping of the paired-end reads on the fu-
sion sequence is performed taking advantage of
Bowtie(Langmead, 2009) tool which parameters
were set in order to report for a data read only
the best alignment identified. Outputs were later
converted using the Samtools(Li, 2009) and the
BedTools(Aaron, 2009) utilities to obtain suitable
format files for the following phases of the pipeline.
Gene fusions not supported by a threshold number of
paired-end reads will be not considered in the next
steps of the flow.
Fourth Filtering Stage: PCR and Mouse Mates
Filtering.
All the mates mapped onto a specific fusion sequence
have been analyzed in this phase with the main pur-
poses of removing those deriving from PCR artifacts
BIOINFORMATICS2014-InternationalConferenceonBioinformaticsModels,MethodsandAlgorithms
144

and those mapping also on the murine DNA. For what
is concerning PCR artifacts, as depicts in Figure 3, if
more than a mate is mapped in the same location, only
one mate of the group is considered for further analy-
ses (green mates in Figure 3 a).
Figure 3: PCR artifacts removal filter. In Subfigure a is
reported an example of mates deriving from PCR artifacts,
characterized by the same start and end mapping positions
on the fusion sequence. Only one read for group (the green
ones) is maintained for subsequent analyses. In Subfigure
b are reported instead some mates mapped in unambiguous
ladder-like pattern on the fusion sequence. All the mates are
retained in this case for further examination.
As shown in Figure 4 a only the mates survived to
the PCR artifacts removal filter have been mapped
using Bowtie(Langmead, 2009) in single-end mode
on the mouse genome in order to identify those
mates mapped also on this reference. These mates,
that probably derives from murine stromal cells
sequencing (Figure 4 b), are removed from the list
of mates supporting the specific gene fusion (green
mates in Figure 4).
Figure 4: Mouse artifacts removal filter. In Subfigure 4 a are
shown the input mates for the murine filter (all the mates not
deriving from PCR artifacts) that have been mapped onto
the fusion sequence. Mates belonging to such a group that
have been mapped also on the murine genome, as shown in
Figure 4 b (green mates), are not considered in the following
phases of the pipeline.
Fifth Filtering Stage: Paired-end Reads Recon-
struction and Spanning Reads Identification.
After the last described stage of filtering each gene
fusion results supported by a list of single mates in-
stead of the initial paired-end reads obtained from
bowtie run on the fusion sequence. As proven by
Maher et colleagues(Maher, 2009) paired-end reads
are however more meaningful for gene fusions de-
tection so, starting from the list of single mates sup-
porting a putative chimeric transcript the paired-end
read, if present, is reconstructed (green reads in Fig-
ure 5). Mates not belonging to a paired-end reads are
discarded. Among these reads of great importance
are the spanning reads, those reads characterized by
the mapping of the mates on the two different partner
genes of the chimeric transcript. Only those paired-
end reads (spanning reads) having the two mates par-
tially or totally mapped onto the two different partner
genes were retrieved for further examination (yellow
reads in Figure 5). Gene fusions not supported by
a certain number of spanning reads that will be dis-
cussed in Results Section were discarded.
Figure 5: Paired-end and spanning reads representation. In
Figure are reported respectively in green and yellow colours
the paired-end and the spanning reads.
Sixth Filtering Stage: Split Reads Retrieving and
Interesting Fusions Identification.
In this phase, in order to guarantee once again the reli-
ability of both the partner genes involved in the fusion
and the breakpoint coordinates on the same partner
genes, the search for split reads is performed on the
remaining putative chimeric transcripts: Split reads
are indeed capable to account for a base-pair reso-
lution of the gene fusion sequence in the breakpoint
region. This is the reason for which only those span-
ning couples, represented with yellow bars in Figure
6, having one or both the mates mapped in the break-
point region provided by Chimerascan(R.Iyer, 2011)
and deFuse(McPherson, 2011) tools are considered.
The mates belonging to such a couple are called split
mates and are represented in a dashed box in Figure 6.
PCR validation will be performed for those gene fu-
sions characterized at least by a threshold number of
split mates discussed once a time in Results Section.
Figure 6: Split mates representation. In Figure are reported
some spanning paired-end reads. The mates belonging to
such couples that are mapped in the breakpoint region are
called split mates and are represented in a dashed box.
3 RESULTS
The run of deFuse(McPherson, 2011) on the eleven
metastatic CRC samples detected 132 gene fusions
whereas that of Chimerascan(R.Iyer, 2011) 167. The
two different outputs didn’t show overlapping fu-
sions as was initially supposed considering the re-
ANovelPipelineforIdentificationandPrioritizationofGeneFusionsin
Patient-derivedXenograftsofMetastaticColorectalCancer
145

cent researches before mentioned(Abate, 2012)(Car-
rara, 2013).These results, not so conspicuous if con-
sidered the number of samples under examination and
the mean amount of gene fusions usually identified
in RNA-Seq samples, are essentially due to the poor
coverage of the samples. It is worth noting how-
ever that generally higher the coverage of the sam-
ples higher will be the number of detected gene fu-
sions with remarkably computational costs spent for
the detection. It is at the same time worth noting that
not all the 299 fusions can be tested in lab using PCR
for known economic and temporal restraints. The pro-
posed pipeline was therefore applied in order to pri-
oritize the identified 299 chimeric transcripts. In the
following we refer to the different chimeric transcripts
using couples of capital letters (actual gene names
cannot be disclosed as the biological results of this
research are currently under review).
For what is concerning the first filtering stage a
threshold of one split read was imposed: Thirteen
gene fusions were here selected because supported
by at least one split read and present at least one
of the other features described in Subsection First
filtering stage: Gene fusions annotation and selec-
tion. The two thresholds were selected with the in-
tention to be as preservative as possible in evaluat-
ing the different candidates. These parameter val-
ues can be however tuned in order to satisfy spe-
cific needs. Furthemore even if among the initial 299
fusion genes none have been previously detected in
cancer samples, all the genes involved in the thire-
teen fusions are characterized by mutational states re-
lated to cancer development and progression (infor-
mation deriving from literature sources and COSMIC
database(Simon, 2010)). Three out of the thirteen
chimeras that passed the first filtering stage are more-
over characterized by a partner gene found to be fused
with other genes in cancer diseases.
For each of these chimeric transcripts, the fusion
sequence has been then retrieved and analyzed in or-
der to understand the biological mechanism at the
basis of the recombination. The criteria of the sec-
ond filtering stage reduced the previous list to only
eight gene fusions characterized by the presence of a
Kozac sequence (or an ATG triplet) at 5’-end or by
an in frame configuration. Also frame shifted con-
figurations could however be interesting in case of
tumor suppressors 3’ partner genes: In the proposed
pipeline this scenario has not been considered because
no oncogenic suppressor genes were detected among
the identified chimeric transcript partner genes.
In Table 1 are reported the results relative to the
third and fourth filtering stages. In particular, a
threshold of at least one paired-end read was fixed
in order to select a fusion for the next phases of the
pipeline being as much preservative as possible: Only
one gene fusion (i.e gene fusion G-H) was deleted
because not supported by paired-end reads as shown
in column 3 of Table 1. The differences among
the breakpoint sequence lengths (Column 2 of Ta-
ble 1) can be attributed to the fact that they depend
on the number of reads used to define the fusion
sequence. So higher the number of reads mapped
by the chimeric transcript discovery tool on the sup-
posed breakpoint sequence, higher will be the pro-
vided length of the same sequence and the probability
of finding with the propose pipeline paired-end reads
aligning on the same. The absence of mates removed
by the mouse remove filter, shown in Column 5 of
Table 1, confirm the fact that effectively the samples
were composed exclusively of human tumor cells. On
the other and, instead, the PCR in the most of cases
caused a remarkably number of artifacts, as it is pos-
sible to note from Column 4 of Table 1.
After PCR and mouse mates removal, for each
of the remaining seven gene fusions the supporting
paired-end reads, if present, are reconstructed. This
activity is followed by the identification of the so
called paired-end spanning reads if existing. The re-
sults are shown in columns 2 and 3 of Table 2.
Two of the previous seven gene fusions have been
removed because they are not supported by spanning
reads (i.e. gene fusions I-L and M-N). A threshold
of one spanning read was indeed imposed in order to
consider a gene fusion. The value selected derives, as
already largely discussed, from the desire to be very
preservative since the previous filtering stages con-
cerning functional and biological properties of fusion
genes have been already capable as shown to remove a
conspicuous number of not functional chimeric tran-
scrips. It is worth noting however that it is possible
to set this parameter according to the specific require-
ments.
The fourth column of Table 2 reports instead the
number of split mates supporting the remaining five
chimeric transcripts. In the last filtering stage a
threshold of at least one split mate was imposed in
order to consider a gene fusion for in lab validation.
Even for the split reads the value parameter was se-
lected, as already said in relation to spanning reads
threshold, in order to be as conservative as possi-
ble. Of the initial 299 gene fusions at the end of
the pipeline five were considered priority (i.e gene fu-
sions A-B, C-D, E-F, O-P and Q-R). Three out of five
have been actually validated in lab using PCR result-
ing as true gene fusions. In table 3 is reported a sum-
mary of the number of fusion genes obtained after the
application of the different filtering stages.
BIOINFORMATICS2014-InternationalConferenceonBioinformaticsModels,MethodsandAlgorithms
146
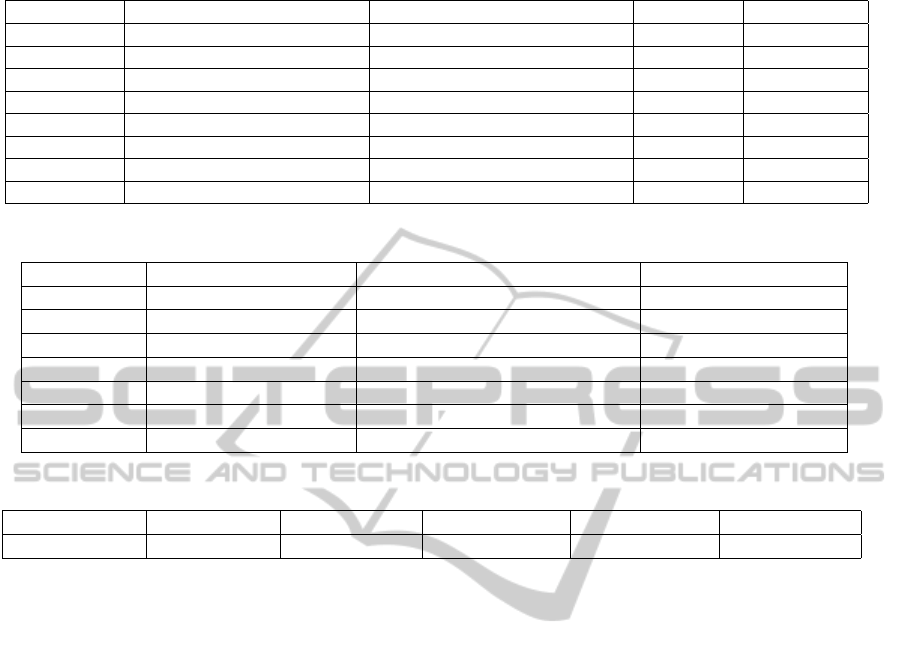
Table 1: Third and Fourth Filtering Stages results.
Gene Fusion Gene Fusion sequence length Nr of mapped paired-end Reads PCR Mates Mouse Mates
A-B 3668 767 529 0
C-D 127 66 74 0
E-F 168 1 0 0
G-H 139 0 0 0
I-L 599 58 28 0
M-N 311 10 4 0
O-P 1886 32 2 0
Q-R 531 86 58 0
Table 2: Fifth and Sixth Filtering Stages results.
Gene Fusion Nr of paired-end Reads Nr of Spanning paired-end reads Number of Split Mates
A-B 344 2 2
C-D 16 11 12
E-F 1 1 2
I-L 18 0 0
M-N 6 0 0
O-P 30 2 3
Q-R 43 5 8
Table 3: Number of chimeric transcripts obtained as output of the different FSs (Filtering Stages).
#Initial fusions #Fusions I FS #Fusions II FS #Fusions III FS #Fusions IV FS #Fusions V FS
299 13 8 7 5 5
4 CONCLUSIONS AND FUTURE
WORK
Starting from the 299 gene fusions detected by
Chimerascan(R.Iyer, 2011) and deFuse(McPherson,
2011) on the eleven metastatic CRC xenopatients
RNA-Seq samples, the proposed pipeline was able to
progressively reduce the list of the identified chimeric
transcripts. Five gene fusions in particular passed all
the filtering stages and were considered to be rele-
vant. The in lab validation of three of these gene fu-
sions confirmed the presence of a chimeric transcript
product proving that the developed pipeline is able to
identify true chimeric transcripts potentially associ-
ated to cancer onset and progression. Furthermore all
the activities performed within the pipeline have been
implemented considering the general features at the
basis of a real, productive and biologically relevant
gene fusion making this program capable to priori-
tize gene fusions from PDXs affected also by differ-
ent diseases. Surely the proposed methodology can be
transferred to other RNA-Seq dataset deriving from
PDXs even with different pathologies. All the activ-
ities performed within the pipeline have been indeed
implemented considering the general features at the
basis of a real, productive and biologically relevant
gene fusion making this program capable to prioritize
gene fusions from PDXs affected as said by different
diseases. We would like to underline that our aim is
just to apply as soon as possible the proposed method-
ology o other dataset in order to improve the filtering
stages with new features and at the same time to make
very user friendly the entire tool.
Future works will aim at: (i) validate all the detected
fusions using also Real-Time PCR (RT-PCR), (ii) in-
tegrate in the proposed pipeline results from other
chimeric transcript detection tools, (iii) change the
setting of the implemented filters in order to evalu-
ate the filtering stages performances, (iv) investigate
the functional role of the identified fusion transcripts,
(v) evaluate the occurrence of the detected fusions in
public RNA-seq dataset (TCGA).
REFERENCES
Aaron, R. (2009). Bedtools: a flexible suite of utilities for
comparing genomic features. Bioinformatics.
Abate, F. (2012). Bellerophontes: an rna-seq data analysis
framework for chimeric transcripts discovery based on
accurate fusion model. Bioinformatics.
Aman, P. (1999). Fusion genes in solid tumors. Semin
Cancer Biology.
ANovelPipelineforIdentificationandPrioritizationofGeneFusionsin
Patient-derivedXenograftsofMetastaticColorectalCancer
147

Ansorge, W. (2009). Next-generation dna sequencing tech-
niques. New Biotechnology.
Bertotti, A. (2011). A molecularly annotated platform of
patient-derived xenografts (’xenopatients’) identifies
her2 as an effective therapeutic target in cetuximab-
resistant colorectal cancer. Cancer Discovery.
Carrara, M. (2013). State-of-the-art fusion-finder algo-
rithms sensitivity and specificity. BioMed Research
International.
Conway, T. (2012). Xenome–a tool for classifying reads
from xenograft samples. Bioinformatics.
Cunningham, D. (2010). Monoclonal antibodies in the
treatment of metastatic colorectal cancer: a review.
Colorectal Cancer.
Edwards, P. (2010). Fusion genes and chromosome translo-
cations in the common epithelial cancers. The Journ.
of Pathology.
Hanahan, D. (2000). The hallmarks of cancer. Cell.
Langmead, B. (2009). Ultrafast and memory-efficient align-
ment of short dna sequences to the human genome.
Genome Biology.
Li, H. (2009). The sequence alignment/map (sam) format
and samtools. Bioinformatics.
Maher, C. (2009). Chimeric transcript discovery by paired-
end transcriptome sequencing. PNAS.
Mardis, E. (2008). The impact of next-generation sequenc-
ing technology on genetics. Trends in genetics.
McPherson, A. (2011). defuse: An algorithm for gene fu-
sion discovery in tumor rna-seq data. PLOS Compu-
tational Biology.
Metzker, M. (2010). Sequencing technologies - the next
generation. Nature Reviews Genetics.
Mitelman, F. (2007). The impact of translocations and gene
fusions on cancer causation. Nature Reviews.Cancer.
Ozsolak, F. (2011). Rna sequencing: advances, challenges
and opportunities. Nat Rev Genet.
R.Iyer (2011). Chimerascan: a tool for identifying chimeric
transcription in sequencing data. Bioinformatics.
Rossello, F. (2013). Next-generation sequence analysis of
cancer xenograft models. PLoS ONE.
Siena, S. (2009). Biomarkers predicting clinical outcome
of epidermal growth factor receptortargeted therapy in
metastatic colorectal cancer. JNCI.
Simon, A. (2010). Cosmic: mining complete cancer
genomes in the catalogue of somatic mutations in can-
cer. Nucleic Acids Research.
Valdes, C. (2013). Characteristics of cross-hybridization
and cross-alignment of expression in pseudo-
xenograft samples by rna-seq and microarrays.
Journal of Clinical Bioinformatics.
Walther, A. (2000). Genetic prognostic and predictive
markers in colorectal cancer. Nature Reviews.Cancer.
BIOINFORMATICS2014-InternationalConferenceonBioinformaticsModels,MethodsandAlgorithms
148
