
Can Simple Electronic Instrumentation Associated with Basic
Training Help Users of Assistive Devices?
Presenting and Verifying the Effectiveness of a Biofeedback Module for an
Instrumented Crutch
Renato Varoto
1
, Aline Midori Rodrigues Sato
1
, Carolina Lins
2
and Alberto Cliquet Jr.
1,2
1
Department of Electrical Engineering, University of São Paulo (USP), São Carlos, Brazil
2
Department of Orthopedics and Traumatology, University of Campinas (UNICAMP), Campinas, Brazil
Keywords: Crutches, Biofeedback, Rehabilitation.
Abstract: Crutches are prescribed towards compensating lower limb overload and adding sensory information through
upper limb. However, adequate loads are required to avoid upper limb lesions and further lower limb
injuries. Therefore, this work describes the development and application of a biofeedback module for a
Lofstrand crutch, based on a simple electronic instrumentation. The goal is to train the user to apply proper
load on the crutch. Basic training was performed by healthy subjects before and after static and dynamic
activities. Results showed the feasibility of the device and the effectiveness of the training to reach the
target (load on the crutch of 20% of body weight).
1 INTRODUCTION
The most active form of human mobility is gait,
being characterized by the gait cycle.
Gait cycle
(stride) is the continuous repetitive pattern of
walking or running, including stance (single and
double supports) and swing phases.
It starts when
one foot makes contact with the floor and ends when
the same foot makes contact again
(Agarwal et al.,
2012; Simoneau, 2011; Wall, 2001)
. During the
stance phase,
the foot is in contact with the floor;
and the leg moves freely above the floor in the
swing phase (Baker, 2012).
Assistive devices for mobility are prescribed to
compensate orthopaedics problems such as pain,
joint instability and lower limb overload (Cook and
Hussey, 2002).
In addition to reducing the load on the lower
limbs, the crutches are used towards increasing the
support base, adding sensory information and
allowing acceleration control during the gait (Saad,
2007; Delisa and Gans, 1983). Applied loads less
than 20% of body weight of the user are adequate
for this device (Chen et al., 2001; Melis et al., 1999).
To verify loads on the crutch, Leite and Cliquet
(2002) developed a system based on the
instrumented Lofstrand crutch and user friendly
software to analyse and save the data. The crutch
was instrumented with strain gauges, being
characterized by threshold of 105N. The system was
validated with force plate equipment, and
simultaneous measurements using both systems
present values with correlation of 0.98.
This paper describes the development and
application of a biofeedback module for the
instrumented Lofstrand crutch described previously.
This device based on simplistic electronic
instrumentation and coupled to the crutch sends an
audio signal when the user exerts more than 20% of
body weight on the crutch. The aim is to familiarize
the user with the proper load, thus avoiding upper
limb lesions and further lower limb injuries. To
verify the effectiveness of the biofeedback module,
healthy subjects performed rapid training and
executed pilot trials based on static and dynamic
activities.
2 MATERIALS AND METHODS
This work was done at the Laboratory of
Biocybernetics and Rehabilitation Engineering -
USP and at Laboratory of Biomechanics and
259
Varoto R., Midori Rodrigues Sato A., Lins C. and Cliquet Jr. A..
Can Simple Electronic Instrumentation Associated with Basic Training Help Users of Assistive Devices? - Presenting and Verifying the Effectiveness of
a Biofeedback Module for an Instrumented Crutch.
DOI: 10.5220/0004914502590264
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2014), pages 259-264
ISBN: 978-989-758-013-0
Copyright
c
2014 SCITEPRESS (Science and Technology Publications, Lda.)

Rehabilitation of the Locomotor System -
UNICAMP. Instrumentation was designed at USP,
and pilot trials performed by healthy subjects were
carried out on both laboratories.
The instrumented Lofstrand crutch has four
strain gauges in Wheatstone bridge configuration,
compensating temperature variation. The voltage
across the centre of the bridge is applied to
instrumentation amplifier, assuring adequate range
of signal and isolation of measurement circuit.
Besides, the instrumentation amplifier presents rail-
to-rail output (range of 4.8V) and makes the
connection between the bridge and the biofeedback
module (Leite and Cliquet, 2002).
2.1 Biofeedback Module
The main components of the biofeedback module
are microcontroller, binary-coded decimal (BCD) to
7-segment decoder, 8-bit monolithic digital-to-
analog converter (DAC), comparator circuit and
non-retriggerable monostable multivibrator (Figure
1).
Figure 1: Block diagram of the biofeedback module,
including the instrumented Lofstrand crutch.
The microcontroller used was PIC16F84
(Microchip Technology Inc., Chandler, AZ, USA)
and it was programmed to determine the value
corresponding to 20% of body weight (N) from body
mass (kg) of the crutch user. Furthermore, through
the calibration equation of the crutch, this value is
converted into a digital electrical signal; and then,
applied to the DAC.
The comparator circuit, which used an
operational amplifier as active component, receives
electrical signals from the crutch instrumentation
amplifier and the DAC. It compares the desired load
exerted on the crutch with the actual load and, if the
load on the crutch is greater than the desired one for
longer than 1s, the multivibrator is activated.
Based on the 555 monolithic timing circuit, the
non-retriggerable monostable multivibrator was
configured to generate an audio signal with duration
of 1s.
The whole electronic circuit, including the
original circuit of the crutch, is powered by two
rechargeable batteries (9V, 150mAh).
2.2 Pilot Trials
Five healthy subjects were recruited to participate in
this study (Table 1). Inclusion criteria were body
mass above 50kg and normal gait pattern. Exclusion
criteria were based on the presence of any upper
extremity musculoskeletal disorders, and not being
able to understand the instructions for the trials.
Subject C had no experience with assistive devices
for ambulation, and others had previous experience
(less than 3 months of use). Informed consent and
Ethical Committee approvals were obtained.
Table 1: Subjects characteristics.
Subjects Gender Age (year) Body mass (kg)
A
M 23.9 80.1
B
M 22.7 76.7
C
F 22.0 68.6
D
M 23.7 73.9
E
M 26.1 82.4
For each subject, before initiating the trials, the
body mass was determined using a bathroom scale
equipped with high precision sensor (Accumed
Produtos Médico Hospitalares Ltda., Duque de
Caxias, RJ, Brazil). The crutch was fitted according
to the user height, such that the handle was
approximately at the level of the greater trochanter,
leaving the elbow flexed about 30
o
(Edelstein, 2013;
Moriana et al., 2013; Laufer, 2003). Thus, the use of
the crutch is not influenced by user height.
Pilot trials were based on two activities (static
and dynamic) acquiring force values on the crutch,
and a period of training using the biofeedback
module. Each activity was repeated 3 times. Left
lower limb injury was simulated by the subjects;
thus, they used the crutch on the right forearm
(contra lateral side) (Melis et al., 1999). For all
trials, subjects were instructed to exert 20% of body
weight on the crutch.
During static activity, the subjects remained
standing, with the feet aligned. The tip of the crutch
was 100mm lateral and 150mm anterior to the right
foot (Edelstein, 2013). This activity lasted 10s, and
marks were put on the floor to help the subject and
standardize the trials (Figure 2).
BIODEVICES2014-InternationalConferenceonBiomedicalElectronicsandDevices
260
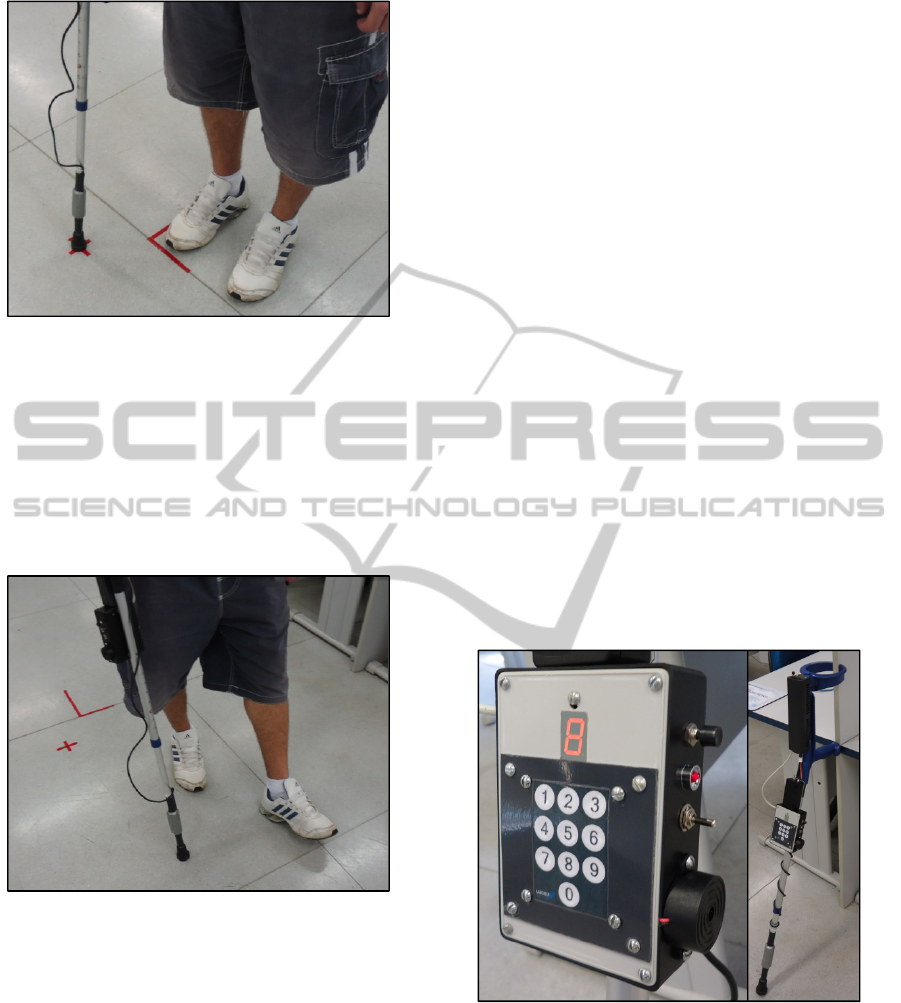
Figure 2: Subject during static activity.
The subjects performed a route of 8m in straight
line aided by crutch during dynamic activity. They
were instructed to advance the crutch and the pseudo
injured lower limb (left) and then step forward with
the healthy lower limb (right), with simultaneous
contra-lateral support of heel and crutch (Saad,
2007) (Figure 3). Gait speed was a free choice,
according to the natural pattern of the subjects.
Figure 3: Subject during dynamic activity.
The period of training was based on the use of
the biofeedback module, in order to familiarize the
subject towards applying 20% of body weight on the
crutch. Thus, the body mass data was entered in the
module, and every time the load exceeded the value
of 20% of body weight, the audible signal was
emitted. The subjects were free to use any training
strategy for as long as they felt like, not exceeding 5
minutes for each subject.
After the period of training, static and dynamic
activities were repeated to verify the training
effectiveness.
2.3 Data Processing and Analysis
All acquired data were low pass filtered at 10Hz
(finite impulse response) to smooth the signal.
For static activity, mean and standard deviation
(SD) were calculated for the force values above
105N, and maximum (max) and minimum (min)
forces were determined in relation to 30s of activity
duration. Mean and SD values are related to
accuracy and precision, respectively, i.e., the user's
ability to apply appropriate loads repetitively on the
crutch.
In relation to dynamic activity, the number of
gait cycles (strides) was counted during the whole
route (3x8m). Therefore, the number of gait cycles
corresponds to the number of contacts of the crutch
on the floor. The peak value of forces was
determined for each contact of the crutch and mean
and SD, maximum and minimum of values of peak
above 105N were calculated.
3 RESULTS
Figure 4 shows the biofeedback module and the final
version of the instrumented Lofstrand crutch, whose
mass is 1.1kg.
Figure 4: Biofeedback module and the final version of
instrumented Lofstrand crutch.
On the front panel, the module has a 10-digit
keypad in which the value of body mass of the
crutch user is entered. The module accepts values at
range of 12.0 to 99.9kg. Thus, this value must be
typed with 3 digits, in other words, with a resolution
of 0.1kg. Each digit is shown on the 7-segment
display sequentially.
CanSimpleElectronicInstrumentationAssociatedwithBasicTrainingHelpUsersofAssistiveDevices?-Presentingand
VerifyingtheEffectivenessofaBiofeedbackModuleforanInstrumentedCrutch
261

Table 2: Target forces and forces applied on the crutch during static activity.
Subjects
Before the training
Target [N]
After the training
Min [N] Mean(SD) [N] Max [N] Min [N] Mean(SD) [N] Max [N]
A
▲ ▲ ▲ 157.0 106.4 121.2(7.6) 139.9
B
▲ ▲ ▲ 150.3 107.3 125.9(9.8) 146.0
C
▲ ▲ ▲ 134.5 ▲ 122.4(13.5) 156.3
D
▲ 117.4(6.5) 135.3 144.8 114.9 132.1(8.3) 153.1
E
▲ 115.4(8.1) 139.8 161.5 ▲ 127.1(19.9) 188.6
The lateral side presents a pushbutton to reset the
microcontroller (if necessary), the buzzer which
receives the output of the non-retriggerable
monostable multivibrator, and a toggle switch to set
one of two functions of the final version of
instrumented Lofstrand crutch: acquiring signals
corresponding to forces applied to the crutch or
training the user with biofeedback. The second
function is independent of the computer, allowing
the user to train anywhere (outside clinical
environment).
In relation to the static and dynamic activities,
the target forces applied on the crutch for subjects A,
B, C, D and E were 157.0N, 150.3N, 134.5N,
144.8N and 161.5N, respectively.
Before the training, during static activity, two
subjects applied forces above 105N, and even then,
the minimum force was not detected. After the
training, all subjects applied forces that were
detected by the crutch. Table 2 presents the force
values for each subject.
For dynamic activity, table 3 presents the number
of gait cycles and the number of detected contacts of
the crutch on the floor.
Table 3: Gait cycles and Subjects characteristics.
Subjects
Before the training
After the training
Gait
cycles
Detected
contacts
Gait
cycles
Detected
contacts
A
21 21 21 19
B
21 15 19 17
C
24 4 24 24
D
22 22 21 21
E
21 21 21 16
Figure 5 shows peak value of forces for each
subject during the dynamic activity, before and after
the training with biofeedback module.
4 DISCUSSION
In relation to the biofeedback module
instrumentation, the use of a microcontroller with an
analog-to-digital converter (ADC) and perform a
comparison with the firmware is a feasible
alternative. However, solid state DAC enables better
adjustment of the parameters coming from the
microcontroller, in this case, the percentage of body
weight of the user. This adjustment, which was done
once, allows to set load limit (based on body weight)
on the crutch through hardware.
The patients that have gone through orthopaedics
surgical procedures are not allowed to put any load
on the operated limb during the first weeks after
surgery, and in the following months they are
required to exert around 20% of body weight on the
operated limb towards bone remodelling due to
piezoelectric effect. Therefore, the use of assistive
devices such as crutches, canes and walkers are
recommended.
Loads from 15% to 50% of body weight can be
applied on crutches (Melis et al., 1999). However, in
relation to dynamic activities, the crutch becomes
unstable when more than 20% of body weight is
applied on the device (considering only one crutch)
(Chen et al., 2001; Melis et al., 1999). Proper loads
avoid upper limbs lesion such as carpal tunnel
syndrome and, at the same time, relief loads on the
hip and on the injured lower limb (Waring and
Werner, 1989; Blount, 1956). Besides, it is
important in the case of lower limb implant of plates
and screws to stabilize bone fracture site during
osteosynthesis in order to avoid the risk of bone
refractures and consequent loosing of the implant.
According to the results, load on the crutch
substantially changed after the training performed
using the biofeedback module. In relation to the
static activity, the load on the crutch increases,
becoming closer to the target; thus, the pseudo
injured limb was preserved without compromising
the upper limb.
Biofeedback training did show improvement on
both accuracy (subjects A, B and E) and precision
(subject E) related to the awareness of the actual
upper limb load.
Pilot trials demonstrated the effectiveness of
training with instrumented Lofstrand crutch and
BIODEVICES2014-InternationalConferenceonBiomedicalElectronicsandDevices
262

biofeedback module for healthy subjects simulating
left lower limb injury. Thus, the application of
training with instrumented crutch becomes feasible
for orthopaedics patients.
5 CONCLUSIONS
Based on simple construction, the biofeedback
module can help subjects to apply more adequate
loads on the crutch through basic training. Such
innovation is a feasible alternative for patients of
outpatient clinic that have gone through
orthopaedics surgical procedures such as implants
towards osteosynthesis.
ACKNOWLEDGEMENTS
We thank the support by grants from São Paulo
Research Foundation (FAPESP) and National
Council for Scientific and Technological
Development (CNPq).
We are grateful to the volunteers, undergraduate
medical students.
REFERENCES
Agarwal, S., Abidin, A. Z., Chattopadhyay, S., Acharya,
U. R., 2012. Engineering interventions to improve
impaired gait: a review. In: Yu, W., Chattopadhyay,
S., Lim, T. C.; Acharya, U. R. Advances in
Therapeutic Engineering. Boca Raton: CRC
PressTaylor & Francis Group, pp. 335-363.
Baker, R., 2012. Clinical gait analysis. In: Winkelstein, B.
A. Orthopaedic Biomechanics. Boca Raton: CRC
Press-Taylor & Francis Group, pp. 419-443.
Blount, W. P., 1956. Don't throw away the cane. The
Journal of Bone and Joint Surgery, 38, pp. 695-708.
Chen, C. L., Chen, H. C., Wong, M. K., Tang, F. T., Chen,
R. S., 2001. Temporal stride and force analysis of
cane-assisted gait in people with hemiplegic stroke.
Archives of Physical Medicine and Rehabilitation, 82,
pp. 43-48.
Cook, A. M., Hussey, Susan, M., 2002. Assistive
technologies: principles and practice. Missouri:
Mosby.
Delisa, J. A., Gans, B. M., 1993. Rehabilitation Medicine -
Principles and Practice. Philadelphia: J. B. Lippincott.
Edelstein, J. N., 2013. Assistive devices for ambulation.
Physical Medicine & Rehabilitation Clinics of North
America, 24, pp. 291-303.
Laufer, Y., 2003. The effect of walking aids on balance
and weight-bearing patterns of patients with
hemiparesis in various stance positions. Physical
Therapy, 83, pp. 112-122.
Leite, F., I. L. 2002. Development of an Electronic Crutch
to Medical Assistance. Master of Science. University
of São Paulo.
Melis, E. H., Torres-Moreno, R., Barbeau, H., Lemaire, E.
D., 1999. Analysis of assisted-gait characteristics in
persons with incomplete spinal cord injury. Spinal
Cord, 37, pp. 430-439.
Moriana, G. C., Roldán, J. R., Rejano, J. J., Martínez, R.
C., Serrano, C. S., 2013. Design and validation of
GCH System 1.0 which measures the weight-bearing
exerted on forearm crutches during aided gait. Gait
Posture, 37, pp. 564-569.
Saad, M., 2007. Meios auxiliares de marcha. In: Greve, J.
M. D. Tratado de Medicina de Reabilitação. São
Paulo: Roca, pp. 330-333.
Simoneau, G. G., 2011. Cinesiologia da marcha. In:
Neumann, D. A. Cinesiologia do Apararelho
Musculoesquelético - Fundamentos para Reabilitação.
Rio de Janeiro: Elsevier, pp. 627-676.
Figure 5: Peak value of forces for the dynamic activity.
CanSimpleElectronicInstrumentationAssociatedwithBasicTrainingHelpUsersofAssistiveDevices?-Presentingand
VerifyingtheEffectivenessofaBiofeedbackModuleforanInstrumentedCrutch
263

Wall, J. C., 2001. Marcha. In: Durward, B. R., Baer, G.
D., Rowe, P. J. Movimento Functional Humano:
Mensuração e Análise. São Paulo: Manole, pp. 93-
105.
Waring, W. P., Werner, R. A., 1989. Clinical management
of carpal tunnel syndrome in patients with long-term
sequelae of poliomyelitis. Journal of Hand Surgery
(American Volume), 14, pp. 865-869.
BIODEVICES2014-InternationalConferenceonBiomedicalElectronicsandDevices
264
