
A Neural Model of Moral Decisions
Alessio Plebe
Department of Cognitive Science, University of Messina, Messina, Italy
Keywords:
Cortical Model, Moral Cognition, Emotion Modeling, Decision Making.
Abstract:
In this paper a neural model of moral decisions is proposed. It is based on the fact, supported by neuroimaging
studies as well as theoretical analysis, that moral behavior is supported by brain circuits engaged more gener-
ally in emotional responses and in decision making. The model has two components, the first is composed by
artificial counterpart of the orbitofrontal cortex, connected with sensorial cortical sheets and with the ventral
striatum, the second by the ventromedial prefrontal cortex, that evaluate representations of values from the
orbitofrontal cortex, comparing with negative values, encoded in the amygdala. The model is embedded in
a simple environmental context, in which it learns that certain actions, although potentially rewarding, are
morally forbidden.
1 INTRODUCTION
Despite the extraordinarily influential role of neural
computation in the investigation of many human be-
haviors and capacities, no neural model for morality
has yet been developed. It is not surprising, since until
recently the coverage of empirical brain information
about moral cognition was scarce and patchy. Since
(Greene et al., 2001) directed neuroimaging studies
explicitly to moral cognition the situation has signifi-
cantly improved, and the current knowledge, although
far from complete, is sufficient for starting a project
of moral modeling. It is the purpose of this work.
It will start to fill a gap inside the current trend in the
study of human morality, where traditional philosoph-
ical speculation has been supplemented by a plurality
of perspectives: from psychology, economics, neu-
roscience, anthropology, sociology. Neural computa-
tion was still missing.
This unparalleled shift in the study of morality
has been described by more than one philosopher as
the “empirical turn” (Nichols, 2004; Doris and Stich,
2005; Prinz, 2008), and several scholars are fostering
an even more radical approach to the science of moral
behavior, rooted in the understanding of the relevant
brain mechanisms (Verplaetse et al., 2009; Church-
land, 2011).
Two of the most important realizations to emerge
from all the empirical studied done so far, are that
there is no unique moral module, and that relatively
consistent set of brain areas that become engaged dur-
ing moral reasoning are also related to emotions, and
decision making (Greene and Haidt, 2002; Moll et al.,
2005; Casebeer and Churchland, 2003).
Decisions are continuously faced by the brain in
everyday life, from simple motor control up to long
term planning, and few of them specifically involve
moral judgments. Even between actions that we may
judge as “wrong” or “good”, to establish a clear cut
between moral norms and social conventions is not a
simple and straightforward task (Kelly et al., 2007).
The theoretical view embraced by this model is neo-
sentimentalism. It is a view within a philosophical
tradition that goes back to (Hume, 1740), which re-
lates moral properties to certain emotions in an essen-
tial way, and construes morality as a set of prescrip-
tive sentiments, where sentiment denotes the disposi-
tion of the subject to the relevant emotion (Nichols,
2004; Prinz, 2008).
For this reason the model here proposed is based
on circuits that encode emotions, and that performs
decisions on value-based representations. While neu-
rocomputational approaches to morality are still lack-
ing, there are indeed a number of existing models
that verge on emotions and decision making, which
have been a guiding reference for the development
here presented. The GAGE model (Wagar and Tha-
gard, 2004) assembles groups of artificial neurons
corresponding to the ventromedial prefrontal cortex,
the hippocampus, the amygdala, and the nucleus ac-
cumbens, in implementing the somatic-marker effect:
encoding of feelings that have become associated
111
Plebe A..
A Neural Model of Moral Decisions.
DOI: 10.5220/0005032001110118
In Proceedings of the International Conference on Neural Computation Theory and Applications (NCTA-2014), pages 111-118
ISBN: 978-989-758-054-3
Copyright
c
2014 SCITEPRESS (Science and Technology Publications, Lda.)

through experience with the predicted long-term out-
comes of certain responses (Damasio, 1994). In the
ANDREA model (Litt et al., 2008) the orbitofrontal
cortex, the dorsolateral prefrontal cortex, and the an-
terior cingulate cortex interact with basal ganglia and
the amygdala in reproducing the human hypersensi-
tivity to losses over equivalent gains (Kahneman and
Tversky, 1979). The overall architecture of these
models shares similarities with those of (Frank and
Claus, 2006; Frank et al., 2007), in which the or-
bitofrontal cortex interacts with the basal ganglia to
produce dichotomic on/off decisions.
The proposed model is made of several simulated
cortical and subcortical areas, described in detail in
§2. It is embedded in a simplified world, which can
be experienced through vision and taste. There are
two possible kinds of objects in the scene, only one
is edible, like a fruit. Collecting fruits is not allowed
everywhere, there are areas where it is forbidden, and
any violation will call into action an angry face, visi-
ble in the scene. Results about the ability of the model
to learn this simple moral rule, and act accordingly,
will be shown in §3.
2 DESCRIPTION OF THE MODEL
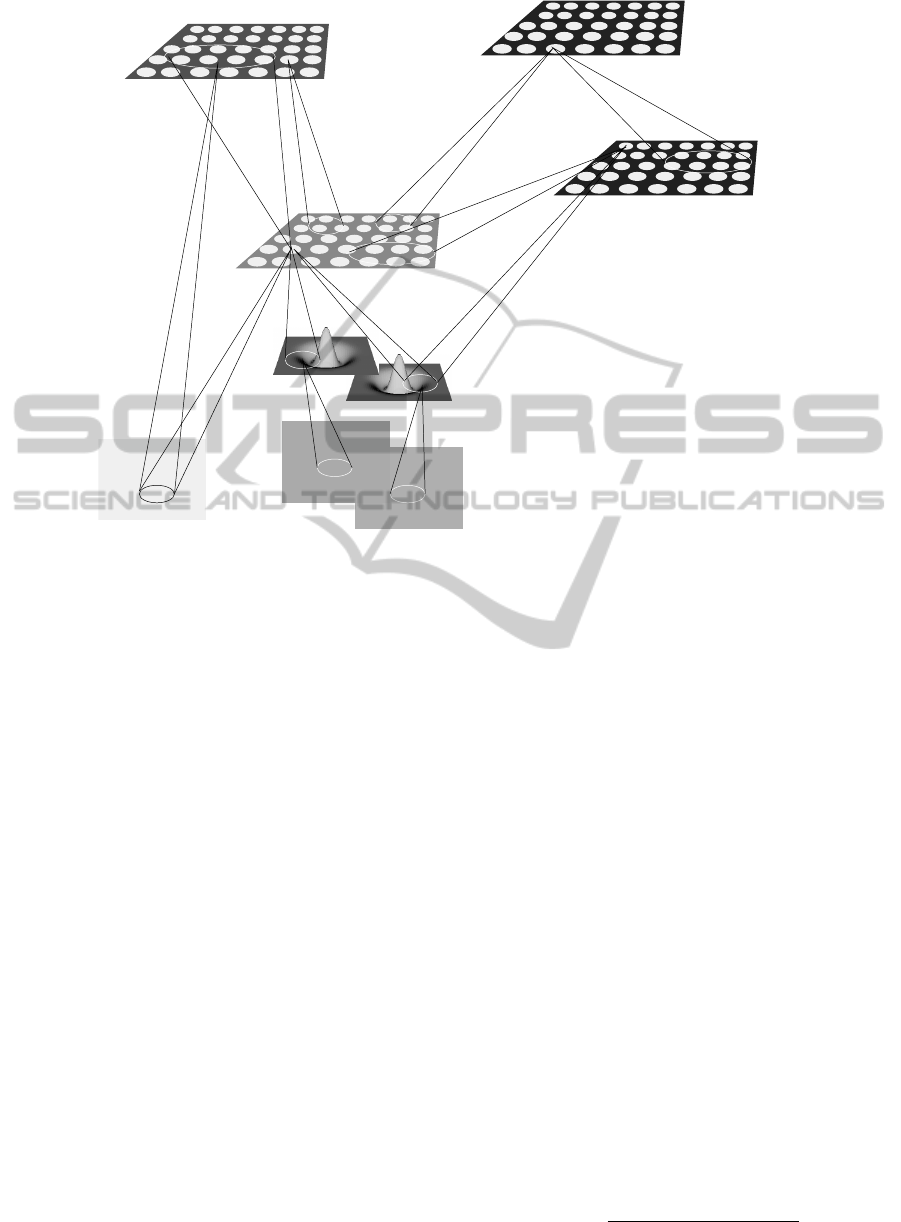
The overall model is shown in Fig. 1. It is composed
by a series of sheets with artificial neural units, la-
beled with the acronym of the brain structure that is
supposed to reproduce. It is implemented using the
Topographica neural simulator (Bednar, 2009), and
each cortical sheet adheres to the LISSOM (Laterally
Interconnected Synergetically Self-Organizing Map)
concept (Sirosh and Miikkulainen, 1997). In a LIS-
SOM sheet of neurons, the activation of each neuron
is due to the combination of afferents and excitatory
and inhibitory lateral connections, as detailed below.
There are two main circuits that learn the emo-
tional component that contributes to the evaluation
of potential actions. A first one comprises the or-
bitofrontal cortex, with its processing of sensorial in-
formation, reinforced with positive perspective values
by the loop with the ventral striatum. The second
one shares the representations of values from the or-
bitofrontal cortex, which are evaluated by the ventro-
medial prefrontal cortex against conflicting negative
values, encoded by the closed loop with the amygdala.
The subcortical sensorial components comprise LGN
at the time when seeing the main scene, the LGN de-
ferred in time, when a possibly angry face will appear,
and the taste information.
2.1 Equations at the Single Neuron
Level
The basic equation of the LISSOM describes the ac-
tivation level x
i
of a neuron i at a certain time step
k:
x
(k)
i
= f
γ
A
~a
i
·~v
i
+ γ
E
~e
i
·~x
(k−1)
i
− γ
H
~
h
i
·~x
(k−1)
i
(1)
The vector fields ~v
i
, ~e
i
, ~x
i
are circular areas of ra-
dius r
A
for afferents, r
E
for excitatory connections,
r
H
for inhibitory connections. The vector ~a
i
is the
receptive field of the unit i. Vectors~e
i
and
~
h
i
are com-
posed by all connection strengths of the excitatory or
inhibitory neurons projecting to i. The scalars γ
A
, γ
E
,
γ
H
, are constants modulating the contribution of affer-
ents, excitatory, inhibitory and backward projections.
The function f is a piecewise linear approximation of
the sigmoid function, k is the time step in the recur-
sive procedure. The final activation of neurons in a
sheet is achieved after a small number of time step
iterations, typically 10.
All connection strengths adapt according to the
general Hebbian principle, and include a normaliza-
tion mechanism that counterbalances the overall in-
crease of connections of the pure Hebbian rule. The
equations are the following:
∆a
r
A
,i
=
a
r
A
,i
+ η
A
x
i
v
r
A
,i
k
a
r
A
,i
+ η
A
x
i
v
r
A
,i
k
− a
r
A
,i
, (2)
∆e
r
E
,i
=
e
r
E
,i
+ η
E
x
i
x
r
E
,i
k
a
r
E
,i
+ η
E
x
i
x
r
E
,i
k
− e
r
E
,i
, (3)
∆i
r
I
,i
=
i
r
I
,i
+ η
I
x
i
x
r
I
,i
k
i
r
I
,i
+ η
I
x
i
x
r
I
,i
k
− i
r
I
,i
, (4)
where η
{A,E,I}
are the learning rates for the afferent,
excitatory, and inhibitory weights, and k · k is the L
1
-
norm.
2.2 Orbitofrontal Circuit
The first circuit in the model learns the positive re-
ward in eating fruits. The orbitofrontal cortex is the
site of several high level functions (Rolls, 2004), in
this model information from the visual stream and
taste have been used. There are neurons in the or-
bitofrontal cortex that respond differentially to visual
objects depending on their taste reward (Rolls et al.,
1996), and others which respond to facial expressions
(Rolls et al., 2006), involved in social decision mak-
ing (Damasio, 1994; Bechara et al., 1994). For (Prehn
and Heekeren, 2009) the role of the orbitofrontal cor-
tex in moral judgment is the representation of the ex-
pected value of possible outcomes of a behavior in
regards to rewards and punishments.
NCTA2014-InternationalConferenceonNeuralComputationTheoryandApplications
112
VS
LGN
taste
vmPFC
Amygdala
OFC
retina
retina’
LGN’
Figure 1: Overall scheme of the model, composed by LGN (Lateral Geniculate Nucleus), V1 (Primary Visual Area), OFC
(OrbitoFrontal Cortex), VS (Ventral Striatum), Amyg (Amygdala), vmPFC (ventromedial PreFrontal Cortex).
In all the subsequent equations the superscript of
time step sk will be omitted, for sake of readability,
and substituted by the name of the sheet, using the
abbreviations L and L
0
for, respectively, the output of
the LGN at the time when seeing the main scene, and
the output of the LGN deferred in time, and the ab-
breviation T for the taste signal.
The equation of the activation of a neural unit in
the OFC layer is the following:
x
(OFC)
= f
γ
(OFC←V1)
A
~a
(OFC←V1)
r
A
·~v
(V1)
r
A
+
γ
(OFC←L
0
)
A
~a
(OFC←L
0
)
r
A
·~v
(L
0
)
r
A
+
γ
(OFC←T)
A
~a
(OFC←T)
r
A
·~v
(T)
r
A
+
γ
(OFC←VS)
B
~
b
(OFC)
r
B
·~v
(VS)
r
B
+
γ
(OFC)
E
~e
(OFC)
r
E
·~x
(OFC)
r
E
−
γ
(OFC)
H
~
h
(OFC)
r
H
·~x
(OFC)
r
H
(5)
which is a specialization of the general equation (1).
Here, for better readability, the unit index i and time
step k have been omitted. There are three sensorial
afferents: ~v
(V1)
r
A
from the visual cortex V1, ~v
(L)
r
A
from
the lateral geniculate nucleus of the thalamus, and
the taste sensorial input ~v
(T)
r
A
, each in a sensorial area
r
A
corresponding to the receptive field of the unit in
OFC. The fourth afferent, ~v
(VS)
r
B
, is the backprojection
from the VS loop that will be described next. The vi-
sual pathway is simplified in a single area, V1, with
the following equation:
x
(V1)
= h
γ
(V1←L)
A
~a
(V1←L)
r
A
·~v
(L)
r
A
+ γ
(V1)
E
~e
(V1)
r
E
·~x
(V1)
r
E
−
γ
(V1)
H
~
h
(V1)
r
H
·~x
(V1)
r
H
(6)
which differs from equation (1) in that the nonlinear
function h has an adaptive threshold θ, dependent on
the average activity of the unit, using:
θ
(k)
= θ
(k)
+ λ
¯x
(V1)
− µ
(7)
where ¯x
(V1)
is a smoothed exponential average in time
of the activity, and λ and µ fixed parameters. This
feature simulates the biological adaptation that al-
lows the development of stable topographic maps or-
ganized by preferred retinal location and orientation
(Stevens et al., 2013). The output of LGN is given
by:
x
(L)
= f
γ
O
~g
(σ
N
)
r
A
−~g
(σ
W
)
r
A
·~v
r,c
β + γ
S
~g
(σ
S
)
r
A
·~x
(L)
S
(8)
ANeuralModelofMoralDecisions
113

approximating the combined contribution of ganglion
cells and LGN with a positive center and negative
surround, by differences of two Gaussian ~g
(σ
N
)
and
~g
(σ
W
)
, with the denominator term acting as contrast-
gain control (Stevens et al., 2013). The bidimensional
coordinates r and c refers to the retinal photorecep-
tors, and ~x
(L)
S
are the suppressive connection field of
the given unit. It holds σ
N
< σ
S
< σ
W
.
OFC has forward and feedback connections with
the Ventral Striatum, VS, which is the crucial center
for various aspects of reward processes and motiva-
tion (Haber, 2011). VS in the model is a crude simpli-
fication of this complex area, and does not reproduce
the details of its direct and reciprocal connection with
the dopaminergic neurons centers. It is implemented
by the following equation:
x
(VS)
= f
γ
(VS←OFC)
A
~a
(VS←OFC)
r
A
·~v
(OFC)
r
A
+
γ
(VS←T)
A
~a
(VS←T)
r
A
·~v
(T)
r
A
+
γ
(VS)
E
~e
(VS)
r
E
·~x
(VS)
r
E
− γ
(VS)
H
~
h
(VS)
r
H
·~x
(VS)
r
H
(9)
The afferent signals ~v
(OFC)
come from equation (5),
~v
(T)
is the taste signal. The output x
(VS)
computed in
(9) will close the loop into the prefrontal cortex with
equation (5).
2.3 Ventromedial Circuit
The second main circuit in the model is based on the
ventromedial prefrontal cortex, vmPFC, and its con-
nections from OFC and the amygdala. The ventrome-
dial prefrontal cortex is long since known to play a
crucial role in emotion regulation and social decision
making (Bechara et al., 1994; Damasio, 1994). More
recently it has been proposed that the vmPFC may
encode a kind of common currency enabling consis-
tent value based choices between actions and goods of
various types (Gl
¨
ascher et al., 2009). It is involved in
the development of morality, in a study (Decety et al.,
2012) older participants showed significant stronger
coactivation between vmPFC and amygdala when at-
tending to scenarios with intentional harm, compared
to younger subjects. The amygdala is the primary
mediator of negative emotions, and responsible for
learning associations that signal a situation as fearful
(LeDoux, 2000). In the model it is used specifically
for capturing the negative emotion when seeing the
angry face, a function well documented in the amyg-
dala (Boll et al., 2011).
vmPFC is implemented in MONE using the stan-
dard equation (1), as follows:
x
(vFC)
= f
γ
(vFC←OFC)
A
~a
(vFC←OFC)
r
A
·~v
(OFC)
r
A
+
γ
(vFC←Amy)
A
~a
(vFC←Amy)
r
A
·~v
(Amy)
r
A
+
γ
(vFC)
E
~e
(vFC)
r
E
·~x
(vFC)
r
E
−
γ
(vFC)
H
~
h
(vFC)
r
H
·~x
(vFC)
r
H
(10)
The afferent signals ~v
(OFC)
come from equation (5),
while~v
(Amy)
, the connection from Amygdala, is given
from the following equation:
x
(Amy)
= f
γ
(Amy←OFC)
A
~a
(Amy←OFC)
r
A
·~v
(OFC)
r
A
+
γ
(Amy←L
0
)
A
~a
(Amy←L
0
)
r
A
·~v
(L
0
)
r
A
+
γ
(Amy)
E
~e
(Amy)
r
E
·~x
(Amy)
r
E
−
γ
(Amy)
H
~
h
(Amy)
r
H
·~x
(Amy)
r
H
(11)
The afferent signals ~v
(OFC)
come from equation (5),
while ~v
(L
0
)
is a direct reading of face from the vi-
sual afferents in the thalamus, delayed in time with
respect to the ordinary visual scene. The activation
given from equation (11) will loop inside the vmPFC
by equation (10).
2.4 Decisions in the Ventromedial Area
A method of analysis has been carried out for the
identification of decisions as population coding of
neural activation in the vmPFC map. Let us introduce
the following function:
x
i
(e) : E ∈ E → R; s ∈ E ∈ E , (12)
that gives the activation x of a generic neuron i in
vmPFC in response to an environmental condition e.
This condition is an instance of a class E, belonging
to the set of all classes of conditions E . In this exper-
iment E =
{
E
1
,E
2
,E
3
}
, where E
1
is the set of situa-
tions where an eatable object is freely available in the
scene, in E
2
a fruit is still in the scene, but forbidden,
in E
3
there is a neutral object in the scene. For a class
E ∈ E we can define the two sets:
X
E,i
=
x
i
(e
j
) : e
j
∈ E
; (13)
X
E,i
=
x
i
(e
j
) : e
j
∈ E
0
6= E ∈ E
. (14)
We can then associate to the class E a set of neurons
in the map, by ranking it with the following function:
r(E,i) =
µ
X
E,i
− µ
X
E,i
r
σ
X
E,i
|
X
E,i
|
+
σ
X
E,i
|
X
E,i
|
, (15)
NCTA2014-InternationalConferenceonNeuralComputationTheoryandApplications
114
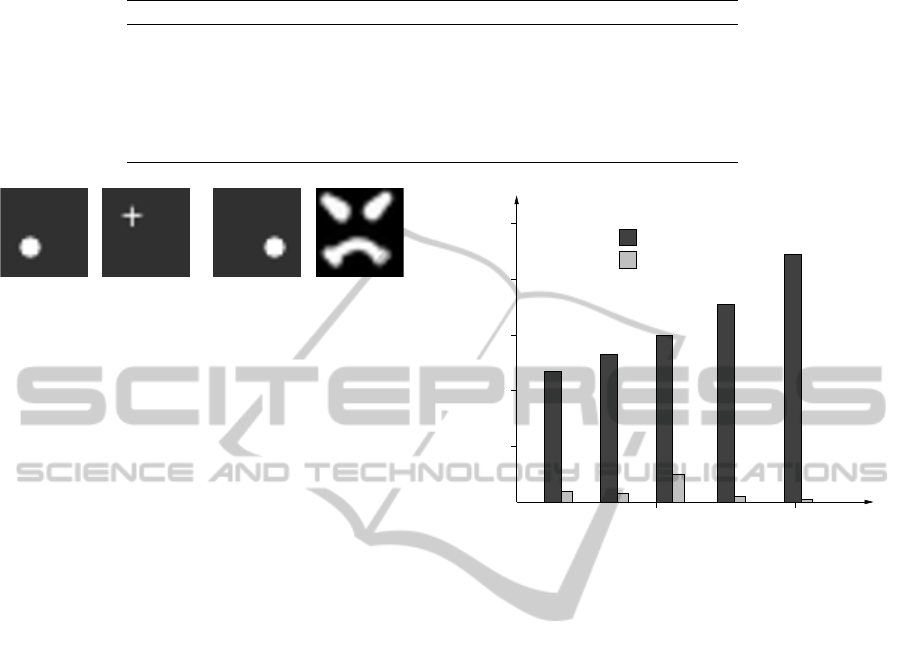
Table 1: Main parameters of all model maps. Radius values are normalized in range [0...2].
layer size r
A
r
A
0
r
E
r
H
γ
A
γ
A
0
γ
E
γ
H
LGN 24 × 24 0.3 - - - - - - - -
V1 22 × 22 0.1 - 0.8 0.4 2.0 - 1.0 0.3
OFC 16 × 16 0.4 0.1 0.1 0.5 0.8 0.4 1.4 1.6
VS 8 × 8 0.5 0.3 0.6 0.3 0.6 0.4 1.4 0.4
Amyg 8 × 8 0.6 0.5 0.5 0.6 0.6 0.3 0.5 1.5
vmPFC 12 × 12 0.4 0.5 0.4 0.2 2.0 −0.5 1.2 1.7
Figure 2: The visual inputs of the model. From the left to
the right: an edible object, possibly an apple, a + shaped
neutral object, the edible object in the forbidden area (the
bottom right quadrant of the scene), which is followed by
the sad and angry schematic face.
where µ is the average and σ the standard deviation of
the values in the two sets, and | · | is the cardinality of
a set. Now the following relation can be established
as the population code of a condition class E:
p(E) : E →
{h
i
1
,i
2
,·· · ,i
M
i
:
r(E, i
1
) > r(E,i
2
) > ·· · > r(E,i
M
)
}
,
(16)
where M is a given constant, typically one order of
magnitude smaller than the number of neurons in the
map The population code p(E) computed with (16) is
used to take a decision d ∈ D according to the status e
of the environment. In this experiment D =
{
d
1
,d
2
}
,
where d
1
is the decision to collect and eat the object,
d
2
is the decision to ignore it.
d(e) = m
argmax
E∈E
(
∑
j=1···E
α
j
x
p(E)
j
(s)
)!
, (17)
where p(E)
j
denotes the j-th element in the ordered
set p(E); α is a constant that is close, but smaller, than
one; m(·) is a mapping function from environmental
categories to decision:
E
1
→d
1
;
E
2
→d
2
;
E
3
→d
2
.
(18)
3 RESULTS AND DISCUSSION
The artificial moral brain architecture just described
is exposed to a series of situations that simulate
highly simplified contexts, and the appropriate ac-
tion is gradually learned. Some actions are charged
plus
apple
% of grasping decisions
learning epochs
20
80
40
60
100
100005000
Figure 3: Percentage of grasping actions selected by the
vmPFC model map, for the apple and the + shaped neutral
object, at different epochs of the development.
with important survival reward, but in some cases may
cause detriment to others. Their angry reaction will
lead to learn that that action is “wrong”.
The main input to the model is a visual scene, ex-
amples are shown in the Fig. 2. Our artificial sub-
ject is unfamiliar with the objects, she can realize how
pleasant fruits are to eat, thanks to its taste perception.
This sensorial input is simply a matrix 2 × 2, in which
the ratio of the upper row to the lower row signal how
pleasant the taste is. Fruits in the bottom right quad-
rant may belong to a member of the social group, and
to collect these fruits would be a violation of her/his
property, that would trigger an immediate reaction of
sadness and anger. This reaction is perceived in the
form of a face with a marked emotion, as the one in
the rightmost position in Fig. 2
3.1 First Learning Stage
This phases of development includes an early stage of
formation of V1, the visual system, with elongated
patterns as inputs, followed by item the good food
recognition stage, in which the stimuli are the two
types of objects, in all possible positions, and their
taste.
Learning is always ruled by equations (2), (3),
ANeuralModelofMoralDecisions
115

20
80
40
60
100
30001000 2000
forbidden
% of grasping decisions
learning epochs
free
Figure 4: Percentage of grasping actions selected by the
vmPFC model map for the apple, placed in the free, or om
the forbidden places, ad different epochs of the develop-
ment.
and (4). applied to the relevant connections. The
development of V1, involving equation (6) only,
setup the main systems of organization in the pri-
mary visual cortex, with arrangement of orientation
tuned neurons, similar to that described in (Plebe and
Domenella, 2007).
Then the OFC, VS, and vmPFC areas of the model
become plastic, and learn their connections of equa-
tions (5), (9), and (10). This set of equations is an
implicit reinforcement learning, where the reward is
not imposed externally, but acquired by the OFC map,
through its taste sensorial input. The amygdala has no
interaction during these stages.
The coding in vmPFC model map is the decision
made to grasp or not to grasp the object, the percent-
age of decision to grasp, at various learning steps, is
shown in Fig. 3. When the object is an apple, grasping
gradually become the prevailing choice, that reaches
to 60% after 5000 learning epochs, and to 90% at the
end of this learning phase. Occurrence of grasping
is instead low for the non rewarding object, and be-
come meaningless, below 5%, at the end of the learn-
ing phase.
3.2 Moral Learning
In this second phase the model receives additional ex-
periences, that of the moral emotion learning, with
the objects as stimuli, followed by an image in which
there could be the angry face. This face will pop up
only when an object of the first kind, the apple, ap-
pears in the right bottom quadrant in the scene. This
is a sort of private property, and the owner reacts with
sadness and anger when his fruit has been grasped.
Now the amygdala gets inputs from both the OFC
map and directly from the thalamus, when the angry
face appears, as from equation (11), and learns its
connections. In this case, there is an implicit rein-
forcement learning as well, with the negative reward
embedded in the input projections to the amygdala.
In Fig. 4 there are the percentages of decisions
to grasp the apple fruit, decoded as before from the
vmPFC map. In this case, the samples of the edible
object have been divided in two groups, depending
on the position in the scene. It can be seen how the
model develops a strong inhibition to grasp the edi-
ble objects when placed in the forbidden sector. At
the end of this development phase the cases of trans-
gression have dropped below 1%. The percentage of
decisions to collect fruits inside the free area of the
scene are always high in all this phase, with values
above 90% at the end of the development. It can be
claimed that the model has learned a moral rule, as an
imperative inhibition to perform certain actions.
3.3 Conclusions
We have described a first attempt to simulate moral
cognition in a neurocomputational model. It has sig-
nificant limitations, and we think its contribution to
the progress of moral science will be modest. First,
the model is able to simulate only one kind of moral
situation, the temptation of stealing food, and the po-
tential consequent feelings of guilt. Since morality
is a collection of several, partially dissociated mech-
anisms, a model must necessarily, at least in its first
implementation, choose a specific one to target. Sec-
ond, even in the single case of stealing, and conse-
quent guilt, the model is missing many brain areas
that are potentially involved, like the cingulate cortex
and the hippocampus, to name few.
Both the design of the moral situation, and the ar-
chitecture of the brain areas, derive from a compro-
mise between manageability of the model, and the
level of knowledge of the functions in brain areas po-
tentially involved. The food stealing situation offers
the advantage of adopting external signals, visual and
of taste, with a well established connections in cru-
cial areas included in the model, like the orbitofrontal
cortex and the amygdala.
While the schematic external world of the model
is a pale resemblance of a typical real situation of hu-
man moral decision, it is a major advance with re-
spect to any existing neural model of decisions. For
example, in the ANDREA model (Litt et al., 2008)
there is only a single input that signals a gain when
positive and a loss when negative; in the models of
Frank and co-workers the input is a combination of
4 possible abstract cues (Frank and Claus, 2006). In
NCTA2014-InternationalConferenceonNeuralComputationTheoryandApplications
116

this model the brain circuits for decisions and emo-
tions have been complemented by a visual system and
a simplified taste input, allowing the simulation of a
schematic worlds where moral relevant events take
place.
Therefore, even in its crudely simplified form, the
model simulates a typical moral situation, using the
relevant stimuli, and plausible neural mechanisms, in
a hierarchy of areas that capture the essence of the
moral decision to be done. We believe that the neu-
rocomputational approach is an additional important
path in pursuing a better understanding of morals, and
this model, despite the limitations here discussed, is a
valid starting point.
REFERENCES
Bechara, A., Damasio, A. R., Damasio, H. R., and Ander-
son, S. W. (1994). Insensitivity to future consequences
following damage to human prefrontal cortex. Cogni-
tion, 50:7–15.
Bednar, J. A. (2009). Topographica: Building and analyz-
ing map-level simulations from Python, C/C++, MAT-
LAB, NEST, or NEURON components. Frontiers in
Neuroinformatics, 3:8.
Boll, S., Gamer, M., Kalisch, R., and B
¨
uchel, C. (2011).
Processing of facial expressions and their significance
for the observer in subregions of the human amygdala.
NeuroImage, 56:299–306.
Casebeer, W. D. and Churchland, P. S. (2003). The neu-
ral mechanisms of moral cognition: A multiple-aspect
approach to moral judgment and decision-making. Bi-
ology and Philosophy, 18:169–194.
Churchland, P. S. (2011). Braintrust – what neuroscience
tells us about morality. Princeton University Press,
Princeton (NJ).
Damasio, A. (1994). Descartes’ error: Emotion, reason
and the human brain. Avon Books, New York.
Decety, J., Michalska, K. J., and Kinzler, K. D. (2012). The
contribution of emotion and cognition to moral sensi-
tivity: A neurodevelopmental study. Cerebral Cortex,
22:209–220.
Doris, J. M. and Stich, S. P. (2005). As a matter of fact:
Empirical perspectives on ethics. In Jackson, F. and
Smith, M., editors, The Oxford Handbook of Contem-
porary Philosophy. Oxford University Press, Oxford
(UK).
Frank, M. J. and Claus, E. D. (2006). Anatomy of a de-
cision: Striato-orbitofrontal interactions in reinforce-
ment learning, decision making, and reversal. Psycho-
logical Review, 113:300–326.
Frank, M. J., Scheres, A., and Sherman, S. J. (2007). Un-
derstanding decision-making deficits in neurological
conditions: insights from models of natural action se-
lection. Philosophical transactions of the Royal Soci-
ety B, 362:1641–1654.
Gl
¨
ascher, J., Hampton, A. N., and O’Doherty, J. P. (2009).
Determining a role for ventromedial prefrontal cortex
in encoding action-based value signals during reward-
related decision making. Cerebral Cortex, 19:483–
495.
Greene, J. D. and Haidt, J. (2002). How (and where) does
moral judgment work? Trends in Cognitive Sciences,
6:517–523.
Greene, J. D., Sommerville, R. B., Nystrom, L. E., Darley,
J. M., and Cohen, J. D. (2001). fMRI investigation of
emotional engagement in moral judgment. Science,
293:2105–2108.
Haber, S. N. (2011). Neural circuits of reward and deci-
sion making: Integrative networks across corticobasal
ganglia loops. In (Mars et al., 2011), pages 22–35.
Hume, D. (1740). A Treatise of Human Nature. Thomas
Longman, London. Vol 3.
Kahneman, D. and Tversky, A. (1979). Prospect theory:
An analysis of decisions under risk. Econometrica,
47:313–327.
Kelly, D., Stich, S., Haley, K. J., Eng, S. J., and
Fessler, D. M. T. (2007). Harm, affect, and the
moral/conventional distinction. Minds and Language,
22:117–131.
LeDoux, J. E. (2000). Emotion circuits in the brain. Annual
Review of Neuroscience, 23:155–184.
Litt, A., Eliasmith, C., and Thagard, P. (2008). Neural affec-
tive decision theory: Choices, brains, and emotions.
Cognitive Systems Research, 9:252–273.
Mars, R. B., Sallet, J., Rushworth, M. F. S., and Yeung,
N., editors (2011). Neural Basis of Motivational and
Cognitive Control. MIT Press, Cambridge (MA).
Moll, J., Zahn, R., de Oliveira-Souza, R., Krueger, F., and
Grafman, J. (2005). The neural basis of human moral
cognition. Nature Reviews Neuroscience, 6:799–809.
Nichols, S. (2004). Sentimental rules: On the natural foun-
dations of moral judgment. Oxford University Press,
Oxford (UK).
Plebe, A. and Domenella, R. G. (2007). Object recognition
by artificial cortical maps. Neural Networks, 20:763–
780.
Prehn, K. and Heekeren, H. R. (2009). Moral judgment
and the brain: A functional approach to the question
of emotion and cognition in moral judgment integrat-
ing psychology, neuroscience and evolutionary biol-
ogy. In (Verplaetse et al., 2009).
Prinz, J. (2008). The Emotional Construction of Morals.
Oxford University Press, Oxford (UK).
Rolls, E. (2004). The functions of the orbitofrontal cortex.
Biological Cybernetics, 55:11–29.
Rolls, E., Critchley, H., Browning, A. S., and Inoue, K.
(2006). Face-selective and auditory neurons in the
primate orbitofrontal cortex. Experimental Brain Re-
search, 170:74–87.
Rolls, E., Critchley, H., Mason, R., and Wakeman, E. A.
(1996). Orbitofrontal cortex neurons: Role in olfac-
tory and visual association learning. Journal of Neu-
rophysiology, 75:1970–1981.
Sirosh, J. and Miikkulainen, R. (1997). Topographic recep-
tive fields and patterned lateral interaction in a self-
organizing model of the primary visual cortex. Neural
Computation, 9:577–594.
ANeuralModelofMoralDecisions
117

Stevens, J.-L. R., Law, J. S., Antolik, J., and Bednar, J. A.
(2013). Mechanisms for stable, robust, and adaptive
development of orientation maps in the primary visual
cortex. JNS, 33:15747–15766.
Verplaetse, J., Schrijver, J. D., Vanneste, S., and Braeck-
man, J., editors (2009). The Moral Brain Essays on the
Evolutionary and Neuroscientific Aspects of Morality.
Springer-Verlag, Berlin.
Wagar, B. M. and Thagard, P. (2004). Spiking Phineas
Gage: A neurocomputational theory of cognitiveaf-
fective integration in decision making. Psychological
Review, 111:67–79.
NCTA2014-InternationalConferenceonNeuralComputationTheoryandApplications
118
