
'Mind Reading': Hitting Cognition by Using ANNs to
Analyze fMRI Data in a Paradigm Exempted from
Motor Responses
José Paulo Marques dos Santos
1,2,3
, Luiz Moutinho
4
and Miguel Castelo-Branco
5
1
University Institute of Maia, Av. Carlos Oliveira Campos, Castêlo da Maia
4475-690 Avioso S. Pedro, Portugal
2
NECE - Research Unit in Business Sciences, University of Beira Interior,
Estrada do Sineiro, 6200-209 Covilhã, Portugal
3
Department of Experimental Biology, Faculty of Medicine, University of Porto
Al. Prof. Hernâni Monteiro, 4200 - 319 Porto, Portugal
4
Adam Smith Business School, University of Glasgow, West Quadrangle,
Gilbert Scott Building, Glasgow G12 8QQ, Scotland, U.K.
5
IBILI (Institute for Biomedical Research on Light and Image), University of Coimbra
Azinhaga Santa Comba, Celas, 3000-548 Coimbra, Portugal
Abstract. The main goal of the present study is to launch the foundations of a
pipeline for fMRI-based human behavior classification, addressing however
some particularities of cognitive processes. While studying cognition, much of
the experiments with fMRI use devices to record subjects’ responses, which re-
cruits the participation of the motor cortex. Although the influence of this as-
pect may be reduced in subtractive univariate analyses methods, it may nega-
tively interfere in multivariate methods. The fMRI data here used is exempted
of motor responses. Subjects were asked to form impressions about persons, ob-
jects, and brands, but their thoughts were not recorded by devices. The feed-
forward backpropagation artificial neural network was used. With this proce-
dure it was possible to correctly classify above randomness. The analysis of the
hidden nodes reveals the extensive participation of the fusiform gyri and lateral
occipital cortex in this cognitive process, corroborating the critical participation
of these structures during classification in the natural brain.
1 Introduction
Although some classifiers have been being proposed for fMRI (functional magnetic
resonance imaging) data analysis [1], and the advantages of such methods have been
already addressed, especially in the study of cognitive processes [2, 3], ANNs have
been missing this trend, with sporadic cross-talks [4-6]. However, ANNs’ advantages
are well known (e.g. modeling non-linear systems) which may be useful in the study
of cognitive processes by modeling and classifying functional data. The present
study’s main goal is to contribute to this developing field.
Since its practical implementation in the mid 90s, fMRI has been extensively used
to better understand human cognition. GLM (General Linear Model) is the mostly
used method to analyze the fMRI signal. However, GLM has some limitations. Its
Marques dos Santos J., Moutinho L. and Castelo-Branco M..
’Mind Reading’: Hitting Cognition by Using ANNs to Analyze fMRI Data in a Paradigm Exempted from Motor Responses.
DOI: 10.5220/0005126400450052
In Proceedings of the International Workshop on Artificial Neural Networks and Intelligent Information Processing (ANNIIP-2014), pages 45-52
ISBN: 978-989-758-041-3
Copyright
c
2014 SCITEPRESS (Science and Technology Publications, Lda.)

univariate nature is one of them. GLM procedure analyzes the signal in a voxel by
voxel basis, i.e. in one voxel independently of any activity in the remaining voxels of
the brain. Nonetheless, there is compelling evidence that processes in the brain unfold
in interconnected networks [7], and neurons’ interdependencies have to be assumed
in order to fully understand cognition. One advantage of multivariated-based methods
is that they consider the activity of all voxels included in the model, which may
emulate brain function closely, at least better than the GLM approach. Comparisons
of these methods may be found elsewhere [8, 9].
The present study explores the applicability of ANNs. ANNs were already
successfully used with fMRI. Misaki and Miyauchi [5] used ANNs to model signal,
although in a procedure similar to the GLM analysis. Recently ANNs were used to
detect Resting State Networks (RSNs) at the individual level [10], acknowledging
that RSNs probably are the most important brain networks discovered with
neuroimaging techniques [11]. Therefore, ANNs may be a suitable method for fMRI
signal analysis, especially in the cognitive domains.
ANNs have a potential advantage in comparison with other multivariate methods.
ANNs’ structure includes nodes in hidden layers which may emulate similar
regularities that exist in the decision process. This facet may be explored in order to
model cognitive processes, especially the complex and multi-stepped ones. Therefore,
the present study uses a proven and effective backpropagation feedforward ANN with
one single hidden layer, in order to favor, more the interpretation of the results (main-
ly the psychological interpretation), than the ANN performance.
One particularity disclosed in [12] is the probable over performance introduced by
motor components when the objective of the study focus on earlier cognitive stages.
Although counterbalancing may reduce motor influences in subtractive univariate
methods, this may not happen in ANNs and the neural activity produced in motor
responses may introduce biases that inflate successful hits. Hence, in this study ANNs
are used to model a cognitive task, but the task is exempted of motor components.
The paradigm is largely inspired in the work of Mitchell, Macrae and Banaji [13].
In their study participants made impressions of persons and objects, two stimuli
classes that have been extensively used in cognitive neuroscience. In the present
study, a third class was added: brand logos. However, in order to explore the
discriminative power of ANNs, this class is split in two: preferred brands and
indifferent brands.
2 Methods
2.1 Paradigm
The structure of the present study relies on the work of Mitchell et al., [13]. It also
includes the same two classes of stimuli, photographs of human faces and objects, but
it adds a third new one: brands’ logos. However, two subclasses of brands’ logos are
considered: preferred and indifferent brands. To disentangle between the two
subclasses, subjects performed a preliminary session where they assessed 200 logos
using the PAD (Pleasure – Arousal – Dominance) scale [14], and
46

the SAM (Self Assessment Manikin) [15]. Fig. summarizes all the assessments.
Fig. 1. Subjects’ aassessments in the preliminary session plotted in the Pleasure - Arousal matrix;
the two rectangles define the selection criteria: the green outline define preferred brands (high
pleasure, high arousal), and the red define indifferent brands (null pleasure, low arousal).
As in [13], the images with stimuli are accompanied with a caption. The caption
includes some information about the subject depicted in the image (person / brand /
object). Participants were instructed to covertly form an impression of the person,
brand, or object taking into account the information in the caption. During the
interstimuli interval participants fixated a cross.
2.2 Data Analysis and Preprocessing
Due to the difficulty in dealing with huge amounts of input data, ANNs have been
used with ROIs (regions of interest) for fMRI signal analysis. ICA (Independent
Component Analysis) may be used in order to previously reduce data dimensionality,
which then allows whole brain analyses [12].
fMRI data pre-processing was carried out using FEAT (FMRI Expert Analysis
Tool) version 5.98, and also using probabilistic independent component analysis
(PICA) [16] as implemented in MELODIC (Multivariate Exploratory Linear
Decomposition into Independent Components) version 3.10, both part of FSL -
FMRIB's Software Library, [17].
Fifteen subjects were randomly assigned to the train group, and the remaining
seven subjects were allocated to the test group.
The fMRI data of the train group entered the PICA analysis for dimension
reduction, which output 173 ICs (independent components). Features were then
extracted from each of the 173 time courses. The strategy adopted was to average the
second and third signals after stimulus onset. By this way, the average time distance
from the onset was 5000 ms, i.e. the signals considered were consistently in the
neighborhood of the hemodynamic response peaks. At the end of this stage the result
is a matrix with 2399 rows (each corresponding to an epoch with the corresponding
47

event), and 173 columns (each corresponding to one IC) plus one more column with
the event code. This matrix is the training set.
The fMRI data of the test group was preprocessed in FEAT. The following pre-
statistics processing was applied: motion correction using MCFLIRT [18]; slice-
timing correction using Fourier-space time-series phase-shifting; non-brain removal
using BET [19]; grand-mean intensity normalization of the entire 4D dataset by a
single multiplicative factor; highpass temporal filtering (Gaussian-weighted least-
squares straight line fitting, with sigma=30.0s). No spatial smoothing was applied.
Registration to high-resolution structural and/or standard space images was done
using FLIRT [18, 20]. All acquisitions were previously registered to a standard brain
(MNI152) in order to make comparisons between subjects possible.
The 173 brain activation maps obtained with the train group were used as masks to
average the individual time courses in the test group. The same procedure for feature
calculation was adopted, i.e. the second and third acquisitions after stimulus onset
were averaged (average time distance from the onset was 5000 ms, equal to the
training set). Finally, the 1119 epochs obtained were normalized for each subject. At
the end of this stage the result is a similar matrix with 1119 rows and 173 columns
(each corresponding to one IC) plus one more column with the event code (that is
used to assess the ANN calculations). This matrix is the training set.
In order to only include input nodes containing critical information for the
classification [21], the 173 ICs were screened. For each IC a GLM was applied. The
timecourse of the IC was the independent variable, and the stimuli onsets convolved
with a gamma function were the explanatory variables. The parameters were
estimated with least mean squares and z statistics computed. The ICs that survived the
screening were those where at least one of the four z was superior to 2.3. Thus, the
ICs screened out had not correlations with the stimuli. This procedure reduced the
quantity of ICs to 82.
2.3 Parameters of the Artificial Neural Networks
The AMORE package [22] implemented in R [23] was used to design and perform
the necessary calculations of the backpropagation feedforward ANN. Exploratory
analyses yielded a global learning rate of 0.07 and a global momentum of 0.8. It was
considered a hidden layer with six nodes. The selected activation function for the
hidden nodes was “tansig”, while for output neurons the function was “sigmoid”.
In order to investigate possible bias derived from the network structure, the ANN
was also fed with a matrix similar to the test set, but now including random values
from a normal distribution. This procedure was completed for 10,000 times in order
to have a large distribution.
3 Results
The results of the ANN with the best performance (more global correct hits) are
represented in Table 1, with the respective accuracies and precisions.
48
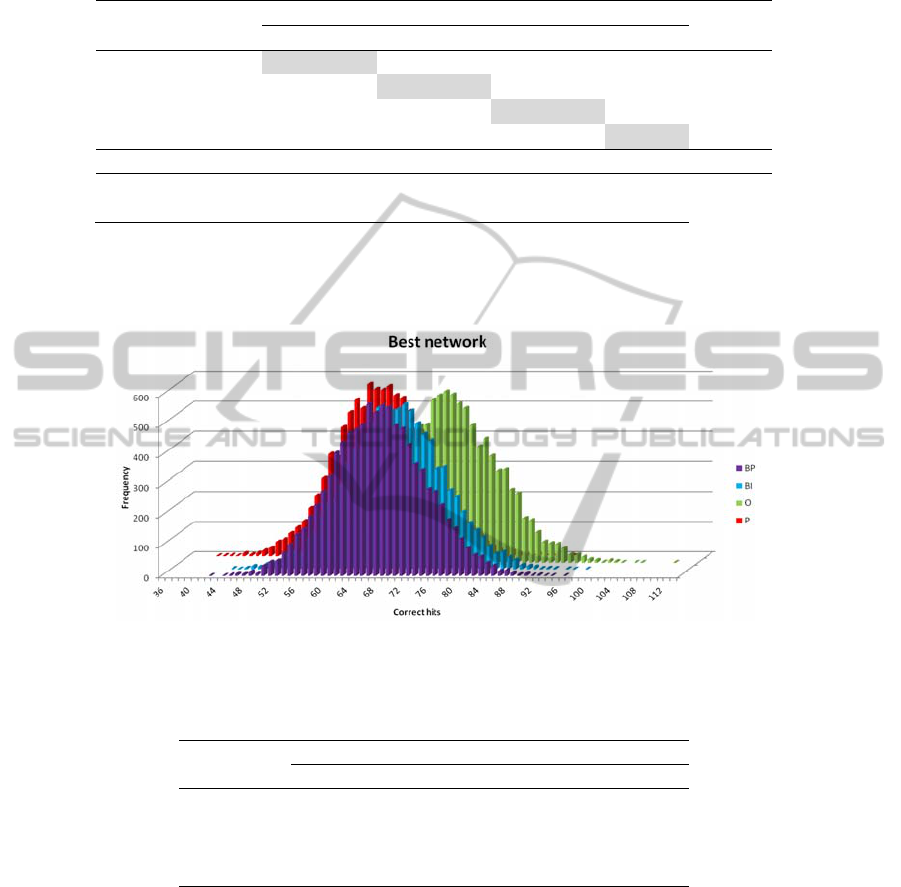
Table 1. Confusion matrix with the predictions of the ANN.
Class
Predicted assessment
Total
BP BI O P
Real
assessment
BP 100 80 45 55 280
BI 91 88 71 30 280
O 49 61 134 36 280
P 59 33 46 141 279
Total 299 262 296 262 1119
Accuracy 33,4% 33,6% 45,3% 53,8%
Precision 35,7% 31,4% 47,9% 50,5%
BP brands preferred; BI brands indifferent; O objects; P persons.
Fig. 1 depicts the results of feeding the network with random values from a normal
distribution. Table 2 represents the probability values of the predictions in Table 1.
Fig. 1. Plot of the results of feeding the network with random values of a normal distribution
(10,000 feeds).
Table 2. Probabilities values of the predictions of the ann based on the distribution obtained
after feeding the network with random normal values for 10,000 times.
Class
Predicted assessment
BP BI O P
Real
assessment
BP 0.000 0.085 1.000 0.942
BI 0.002 0.008 0.794 1.000
O 0.998 0.901 0.000 1.000
P 0.914 1.000 1.000 0.000
BP brands preferred; BI brands indifferent; O objects; P persons.
Table 3 lists the weights of the axons that link hidden nodes to the output nodes. For
the sake of space, the weights of the axons that link input to hidden nodes are not here
fully reported. However, Fig. 2 depicts two axial slices of IC2 and IC7. IC2 has
important positive weights with hidden nodes 1 (10.73) and 5 (22.33) and important
negative weights with hidden node 3 (-10.60) and 4 (-39.56); also IC2 encompasses
voxels in the occipital and temporal occipital fusiform gyrus, and lateral occipital
49

cortex, all bilaterally. IC7 has important positive weights with hidden nodes 1
(13.11), 3 (14.17), and 5 (11.72); IC7 includes voxels from the lateral occipital
cortex, inferior temporal gyrus (temporooccipital part) all bilaterally.
Table 3. Weights of the axons that link hidden to output nodes. The most important positive
weights have blue background, and red for the most important negative weights.
Output
nodes
Hidden nodes
1 2 3 4 5 6
BP 1.569 0.811 1.224 0.288 -1.314 0.679
BI -0.411 -0.680 1.154 -0.838 1.302 0.998
O -0.184 0.983
-1.387 -1.478 1.081 -1.305
P
-1.783 -0.797 -1.151 1.714 -0.966 0.353
BP brands preferred; BI brands indifferent; O objects; P persons.
Fig. 2. Two axial slices (z = -12) of IC2 and IC7; MNI152 coordinates; radiological convention.
4 Discussion
Being 280 the quantity of cases of each class presented to test the network, it would
be expectable that random choice is around 70 (25%) because there are four classes.
In fact the peaks of the distributions in Fig. 1 fall around this value (69 for BP, 71 for
BI, 76 for O, and 63 for P). In Table 2 all the p-values in the diagonal are zero or
close to. The main conclusion of this study is that the basic backpropagation feedfor-
ward ANN with one hidden layer is correctly predicting much above random choice,
i.e. the ANN is extracting critical information from brain data in order to correctly
predict behavioral responses.
It is important to highlight the conditions of this study. Subjects never performed
actions, just made mental impressions about the stimuli. Thus, the ANN is extracting
neural information in the pre-motor stages, supposedly during the perception /
decision periods, which are the most interesting for studies on cognition.
The analysis of the hidden nodes reveals interesting aspects. Hidden node 3 has
important positive weights for preferred and indifferent brands and important
negative weights for objects and persons. Hidden node 5 has important positive
weights for indifferent brands and objects and negative weights for preferred brands
and persons. These two nodes alone are sufficient to discriminate between the four
classes.
50

However, considering hidden node 4, each class (brand, object, and person) is
discriminated. It is possible to conclude that this node is able to successfully
segregate among classes, which is also supported by the data in Table 1 and Table 2.
In fact, the cells with correct hits (grey background) concentrate the majority of the
assessments and have the lowest probability values.
Nonetheless, discriminating between preferred and indifferent brands it is not so
good. The values of the four cells that involve preferred and indifferent brands in
Table 1 are approximate, and in the case of indifferent brands, the network has more
tendency to classify as preferred brands. The analysis of the same four cells in Table
2 confirms this observation. The reason for such has to be explored. The problem
may be intrinsic to the stimulus, because of its low salience, or the method has to be
improved in order to attain such refinement.
IC2 and IC7 (depicted in Fig. 2) are two important sources of data for successful
classification. Interestingly these two brain networks encompass brain regions from
visual and visual associative areas. This is in line with the findings of Hanson,
Matsuka and Haxby [4], which also found in fusiform gyri sources of cognitive data
for accurate classification.
References
1. Pereira, F., Mitchell, T.M., Botvinick, M.: Machine learning classifiers and fMRI: a tutorial
overview. Neuroimage 45, S199-S209 (2009)
2. Mitchell, T. M., Hutchinson, R., Niculescu, R. S., Pereira, F., Wang, X., Just, M., Newman,
S.: Learning to Decode Cognitive States from brain images. Machine Learning 57, 145-175
(2004)
3. Haynes, J.-D., Rees, G.: Decoding mental states from brain activity in humans. Nature
Reviews Neuroscience 7, 523-534 (2006)
4. Hanson, S. J., Matsuka, T., Haxby, J. V.: Combinatorial Codes in Ventral Temporal lobe
for Object Recognition: Haxby (2001) revisited: is there a "face" area? Neuroimage 23,
156-166 2004
5. Misaki, M., Miyauchi, S.: Application of artificial neural network to fMRI regression
analysis. Neuroimage 29, 396-408 (2006)
6. Sona, D., Veeramachaneni, S., Olivetti, E., Avesani, P.: Inferring Cognition from fMRI
Brain Images. In: Marques de Sá, J., Alexandre, L., Duch, W., Mandic, D. (eds.) Artificial
Neural Networks – ICANN 2007, vol.4669, pp.869-878. Springer Berlin/ Heidelberg, 2007
7. Fox, M.D., Snyder, A.Z., Vincent, J.L., Corbetta, M., Van Essen, D.C., Raichle, M.E.: The
human brain is intrinsically organized into dynamic, anticorrelated functional networks.
Proceedings of the National Academy of Sciences of the United States of America 102,
9673-9678 (2005)
8. Pereira, F., Botvinick, M.: Information mapping with pattern classifiers: a comparative
study. Neuroimage 56, 476-496 (2011)
9. Misaki, M., Kim, Y., Bandettini, P.A., Kriegeskorte, N.: Comparison of multivariate
classifiers and Response Normalizations for Pattern-information fMRI. Neuroimage 53,
103-118 (2010)
10. Hacker, C.D., Laumann, T.O., Szrama, N.P., Baldassarre, A., Snyder, A.Z., Leuthardt,
E.C., Corbetta, M.: Resting state network estimation in individual subjects. NeuroImage
82, 616-633 (2013)
11. Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D.A., Shulman,
51

G. L.: A default mode of brain function. Proceedings of the National Academy of Sciences
98, 676-682 (2001)
12. Santos, J. P., Moutinho, L.: Tackling the cognitive processes that underlie brands'
assessments using artificial neural networks and whole brain fMRI acquisitions. 2011 IEEE
International Workshop on Pattern Recognition in NeuroImaging (PRNI), pp. 9-12. IEEE
Computer Society, Seoul, Republic of Korea (2011)
13. Mitchell, J. P., Macrae, C. N., Banaji, M. R.: Forming impressions of people versus
inanimate objects: social-cognitive processing in the medial prefrontal cortex. Neuroimage
26, 251-257 (2005)
14. Russell, J. A., Mehrabian, A.: Evidence for a three-factor theory of emotions. Journal of
Research in Personality 11, 273-294 (1977)
15. Morris, J. D.: Observations: SAM: The self-assessment manikin - an efficient cross-cultural
measurement of emotional response. Journal of Advertising Research 35, 63–68 (1995)
16. Beckmann, C.F., Smith, S.M.: Probabilistic independent component analysis for functional
magnetic resonance imaging. IEEE Transactions on Medical Imaging 23, 137-152 (2004)
17. Smith, S.M., Jenkinson, M., Woolrich, M.W., Beckmann, C.F., Behrens, T.E., Johansen-
Berg, H., Bannister, P.R., De Luca, M., Drobnjak, I., Flitney, D.E., Niazy, R.K., Saunders,
J., Vickers, J., Zhang, Y., De Stefano, N., Brady, J.M., Matthews, P.M.: Advances in
functional and structural MR image analysis and implementation as FSL. Neuroimage 23
Suppl 1, S208-S219 (2004)
18. Jenkinson, M., Bannister, P.R., Brady, J.M., Smith, S.M.: Improved optimization for the
robust and accurate linear registration and motion correction of brain images. Neuroimage
17, 825-841 (2002)
19. Smith, S.M.: Fast robust automated brain extraction. Human Brain Mapping 17, 143-155
(2002)
20. Jenkinson, M., Smith, S.M.: A global optimisation method for robust affine registration of
brain images. Medical Image Analysis 5, 143-156 (2001)
21. Walczak, S., Cerpa, N.: Heuristic principles for the design of artificial neural networks.
Information and Software Technology 41, 107-117 (1999)
22. Limas, M. C., Meré, J. B. O., Marcos, A. G., Ascacibar, F. J. M. d.P., Espinoza, A. V. P.,
Elías, F. A.: AMORE: A MORE flexible neural network package. León (2010)
23. R Development Core Team: R: A Language and Environment for Statistical Computing. R
Foundation for Statistical Computing, Vienna (2010)
52
