
VirtualEnaction
A Platform for Systemic Neuroscience Simulation
Nicolas Denoyelle
1,2
, Florian Pouget
1,2
, Thierry Vieville
1
and Fr
´
ed
´
eric Alexandre
1,2,3
1
Inria Bordeaux Sud-Ouest, 200 Avenue de la Vieille Tour, 33405 Talence, France
2
LaBRI, Universit
´
e de Bordeaux, Institut Polytechnique de Bordeaux, CNRS, UMR 5800, Talence, France
3
Institut des Maladies Neurod
´
eg
´
en
´
eratives, Universit
´
e de Bordeaux, CNRS, UMR 5293, Bordeaux, France
Keywords:
Simulation, Computational Neuroscience, Virtual Reality.
Abstract:
Considering the experimental study of systemic models of the brain as a whole (in contrast to models of one
brain area or aspect), there is a real need for tools designed to realistically simulate these models and to exper-
iment them. We explain here why a robotic setup is not necessarily the best choice, and what are the general
requirements for such a bench-marking platform. A step further, we describe an effective solution, freely avail-
able on line and already in use to validate functional models of the brain. This solution is a digital platform
where the brainy-bot implementing the model to study is embedded in a simplified but realistic controlled
environment. From visual, tactile and olfactory input, to body, arm and eye motor command, in addition to
vital somesthetic cues, complex survival behaviors can be experimented. The platform is also complemented
with algorithmic high-level cognitive modules, making the job of building biologically plausible bots easier.
1 INTRODUCTION
The brain is a fascinating complex structure and de-
signing global models of such a structure is particu-
larly difficult. This is specifically true with regard to
the fact that the brain is a complex system in interac-
tion with the body and the environment. Two impor-
tant consequences can be driven. On the one hand,
modeling the brain includes not only understanding
how each subsystem (visual, motor, emotional, etc.)
works but also how these subsystems interact as a
whole, to yield emerging behaviors, i.e. effects that
result from interactions between subsystems. On the
other hand, studying and validating functional models
of brain structures at a macroscopic scale cannot be
performed with restrained artificial static paradigms
but requires experiments in complex environments,
with realistic sensory-motor tasks to perform, includ-
ing high-level interactive behaviors (e.g. survival
strategy in the presence of prays/predators) and long-
term protocols (since both statistical studies and bio-
plausible learning mechanisms require long epochs).
Such paradigms are to be related to biological ex-
periments conducted on animals. These statements
are not only characterizing brain models, they also
give strong requirements on the tools that must be
designed to simulate these models and to experiment
them.
Designing such tools is also an excellent way to
address at the same time the two main objectives of
such brain models at the macroscopic scale. One the
one hand, they are intended to serve neuroscientists
as a new platform of experimentation, on which they
can apply their classical protocols of observation and
analysis of animals at the behavioral as well as elec-
trophysiological levels. It is consequently important
that neuroscientists can observe the inner activity of
the models, as they use to do for example with elec-
trodes (but we can imagine that this observation in
digital models might be more easy than in the real
brain). It is also important that they can define clas-
sical behavioral protocols like they do in animals (eg.
fear conditioning) in order to observe the resulting be-
havior and the corresponding brain activation. Defin-
ing such protocols implies that the structure of the ex-
ternal world (e.g., maze, food magazine) as well as its
intrinsic rules (eg. tone followed by an electric shock)
be easy to design.
On the other hand, they are also intended to serve
computer scientists as a way to design artificial au-
tonomous systems, driven by the brain models. In
this case, it is important that the supposed properties
of the models (e.g., capacity to learn, robustness to
noise or changing rules) be assessed by rigorous eval-
155
Denoyelle N., Pouget F., Vieville T. and Alexandre F..
VirtualEnaction - A Platform for Systemic Neuroscience Simulation.
DOI: 10.5220/0005166701550163
In Proceedings of the 2nd International Congress on Neurotechnology, Electronics and Informatics (-2014), pages 155-163
ISBN:
Copyright
c
2014 SCITEPRESS (Science and Technology Publications, Lda.)

uation procedures, as it is defined for example in the
domain of machine learning. In this case also an easy
access must be proposed both to the inner circuitry
of the models and to the specification of the external
world.
With in mind this double goal of offering conve-
nient tools to both scientific communities, we report
in this paper the specifications that we have elaborated
and present the corresponding software platform that
we call VirtualEnaction.
2 PROBLEM POSITION
Concerning the nature of such a simulator, real
robotic systems are often used and answer particu-
larly well to the second requirement about a realistic
environment. However, building viable robotic sys-
tems and making them evolve in realistic environ-
ments (e.g. natural sites) for long periods of time
(e.g. several days) is just too expensive in term of
cost and manpower in many circumstances and par-
ticularly during early phases of development. Fur-
thermore, the goal of such simulation is not only to
make a demo, but also, and more importantly, to study
and quantify the behavior of functional models of the
brain. As a consequence we not only need a com-
plex, long-term, realistic experimental setup, but we
also need a controllable and measurable setup where
stimuli can be tuned and responses can be measured.
In fact, real environment complexity and parameters
are intrinsically difficult when not impossible to con-
trol. This is the reason why we propose to use a digi-
tal simulator implementing realistic survival and other
biological scenarios
A step further, available macroscopic models of
brain functions are not designed for ”performance”
but to properly implement phenomenological con-
cepts that have been investigated in some cogni-
tive or behavioral framework. They would therefore
have ”no chance” in a real world. Note that recent
computer science mechanisms designed without any
constraint regarding biological plausibility but only
towards final performances are nowadays probably
more efficient but explain nothing.
As a consequence we also need a setup which can
provide a “simplified environment”, in order systemic
models of the brain at the state of the art not to fail
immediately. We must also take into account the fact
that (i) such models are rather slow to simulate (un-
less huge computer power is available), and that (ii)
they are not supposed to focus on precise issues re-
garding low-level sensory input or motor output but
on integrated cognitive functions and the resulting be-
haviors.
This, in addition to technical constraints, yields
three key characteristics:
1. No real-time but a look-and-move paradigm : The
main restriction we propose to accept here is to
have the simulator running at a “slower” time (i.e.
using several seconds to simulate one real-time
second) and also to consider discrete time sam-
pling. This seems obvious as far as digital sim-
ulation is concerned, but in terms of underlying
framework, this has several consequences (e.g.,
giving up the possibility for a human observer to
interact with the simulation, restraining to clock-
based (and not event-based) dynamical models,
etc.) (Taouali et al., 2011).
2. No real robotic control but only motor command :
Since in the nervous system motor control seems
to be a hierarchical system with high-level motor
commands, while their closed loop execution is
delegated to the peripheral motor system (Uithol
et al., 2012), we may accept to only simulate ges-
ture and displacement at a rather symbolic level
such as “stand-up” or “turn 90
◦
rightward”. This
indeed cancels the possibility to study sharp phe-
nomena of motor interactions with the environ-
ment but allows us to concentrate on high-level
control such as action selection mechanism and
motor planification.
3. Complex but not necessarily natural visual envi-
ronment: The third main restriction we propose
to accept is to consider a complex visual environ-
ment (with visual textures, several objects in mo-
tion, etc.) but not to invest in the simulation of a
realistic natural scene simulation. The reason of
this choice is that natural image vision is an issue
already well studied (Hyv
¨
arinen, 2009). The gen-
eral conclusion is that biological visual systems
are tuned to natural image statistics, decomposed
by the early visual front-end in such a way that
higher-level visual input only relates on cues or-
thogonal (in a wide sense) to natural image statis-
tics. In other words, the job regarding this aspect
is done by early-vision layers and we may con-
sider more stylistic visual cues at a higher-level.
Depending on the study, we may also wish to
work on either a pixelic or a symbolic represen-
tation of the visual scene. See (Teftef et al., 2013)
for details of how the early-visual system relates
both representations.
NEUROTECHNIX2014-InternationalCongressonNeurotechnology,ElectronicsandInformatics
156

3 SYSTEM DESCRIPTION
We consider that a “brainy-bot”, i.e. the implementa-
tion of a global model of the brain functionality, inter-
acts with its environment with the simple goal to sur-
vive. Our objective is to simulate the sensory-motor
interactions of this bot with respect to its environment.
Examples of such surroundings are shown in Fig. 1.
Figure 1: Two examples of digital experimental environ-
ments for systemic neuroscience. Up: A minimal environ-
ment corresponding to a standard maze reinforcement learn-
ing task (source: one of our virtual enaction built). Down:
A complex environment in which survival capabilities are
to be checked (source: landscape encountered when play-
ing with the standard game).
Survival is precisely defined as maintaining vital
variable values in correct ranges, as formalized in,
e.g., (Friston, 2012). In our context, health, food, wa-
ter, energy, and oxygen are the vital state variables.
The bot has access to these values. These variables
decrease or increase with time since the bot body is
supposed to consume the related resource depending
on its activity, or change in the presence of an exter-
nal event (e.g. energy during a predator attack), and
restore resources. Restoring resources is obtained ei-
ther by ingesting items or taking rest (i.e., make the
choice to stop action, with the benefit of vital resource
increase and the drawback of not acting on the en-
vironement, this might be a short-term policy choice
if vital variables are low and is a middle-term policy
choice otherwise).
The environment structure is very simple and
made of “blocks”. Each object in this environment
(including the floor, relief, ..) is a collection of blocks.
Each block is defined by its 3D position, orienta-
tion and sizes, roughness, hardness, temperature, and
color. Some blocks correspond to eatable or drinkable
resources. Other entities correspond to objects that in-
teract with the bot (e.g. predators that attack the bot
or lava to avoid). At this level, survival corresponds
to avoid or kill the predators and find resources to eat
or drink.
The bot anatomy is functionally made of a body,
an arm and an head/eye. It carries a bag with objects
found in its environment. The body can move at a
given speed and in a given direction, and also rotate
at each step to a given angle. It can also jump up to a
given relative height, or knee down to take a rest. The
head/eye can gaze in a given yaw/pitch direction. The
arm can perform predefined symbolic gestures : take
an object in hand out of its bag, put the object in hand
into its bag, either drop or throw the object in hand,
ingest the block in hand (food or water), grasp the
object in front of him. The arm can also attack (quan-
tified by a force value and with or without an object in
hand) the object in front of him. This is the complete
description of the bot motor command output in the
present context.
The bot sensory input corresponds to cues related
to the blocks which are around it. The touch cues al-
low the bot to estimate the roughness, hardness and
temperature of the object in hand. The olfactory cues
allow to estimate the smell type and intensity of ob-
jects close to it (computed by integrating average val-
ues over the blocks characteristics). At the bot level,
pixelic vision provides an image of the visual field
view (i.e., calculating the blocks texture and color
projection on the virtual retina). Finally, the proprio-
ceptive cues correspond to gaze direction estimation.
In order to quantify the bot behavior, the interface
provides an additional access to the bot absolute posi-
tion and orientation in space. Symbolic vision is also
available, as a list of blocks visible in its visual field,
which access to the block characteristics.
An adaptation of the minecraft open game soft-
ware yields the proper answer to this wish-list and the
so called virtualenaction is an open-source free-
license implementation of these specifications. Each
user buys a end-user low-cost mojang license (< 20$)
for minecraft, while virtualenaction is free of
use under a CeCILL-C license. Fully-documented
scripts facilitate the installation of the software bun-
dle under Linux OS. The bot is implemented in ei-
ther C/C++, or possibly in Python (via an existing
swig wrapper) or other computer languages. It uses
a simpleAPI, as described in Fig. 2. Furthermore,
in order to both observe in slow-down real-time the
VirtualEnaction-APlatformforSystemicNeuroscienceSimulation
157

Figure 2: Left: The software interface is trivial: each imple-
mentation of a brainy-bot provides an initialization routine
initBot() and a stop function stopCondition(), while
at each time-step the brainDo() method is called. Right:
All status, input and output functionality are available via
a simple API. For instance, hand or vital input, body and
head displacement, gestures of resource injection and attack
against predator are shown.
bot behavior and interact with the digital experiment,
a graphic user interface is available as described in
Fig. 3.
More details on the computer implementation is
given in Appendix 5.
4 NEUROSCIENCE
APPLICATION
Let us now discuss how this setup constitutes a step
towards integrative neuroscience digital experimenta-
tion.
First of all, let us compare this project with com-
plementary connected projects. The AnimatLab is a
software platform allowing to simulate embodiment
(bio-mechanical simulation of a body) allowing in-
vestigate the relation between brain and body (Cofer
et al., 2010). Furthermore, it proposes a neural net-
work architecture for the implementation of cognitive
function. On the contrary, the present framework has
a rather limited description of the embodiment, but a
much larger set of possible interactions with the en-
vironment. A step further, not only artificial based
neural network models are usable in VirtualEnaction,
whereas the interface with any existing neuroscience
simulation tool (e.g., python based neural simulators,
see (Brette et al., 2007; Davison et al., 2008)) is
straightforward. This feature is essential, since we
must simulate the system at different modeling scale,
as developed now. The Morse is a generic simula-
tor for academic robotics, with realistic 3D simulation
of small to large environments, allowing complete in-
tegration with any simulation tools. It outperforms
concurrent systems like Webot. The interest of Vir-
tualEnaction with respect to Morse is twofold: Since
we target integrative cognitive tasks of survival which
is exactly what happens with the Minecraft environ-
ment, using this specialized product is far simpler
and somehow more demonstrative. In term of per-
formances, as being less sophisticated (using a sim-
plified 3D rendering, while Morse has all 3D capa-
bilities) and being agnostic in terms of programming
languages (i.e., allowing fast C/C++ implementation
of user modules, whereas Morse is limited to Python
scripts) the VirtualEnaction platform is a priori ex-
pected to be more efficient in terms of CPU usage.
However, with a larger humanpower all what has been
developed within VirtualEnaction could have been de-
veloped in Morse.
The main application regarding neuroscience is to
test cognitive computational models in realistic con-
ditions. Very simply, a behavioral experiment is per-
formed on an animal model or on human. Usually
with a training phase, the measurement phase and the
data analysis. In order to formalize the obtained re-
sult a computational model is proposed that explain
the data, and may also have prediction regarding other
falsifiable future experiments. The present software
and methodological tool allow us to propose to en-
hance this very general paradigm in the following di-
rections:
• Test the model prediction for several others ex-
perimental conditions or model parameter ranges :
The idea is to reproduce the experimental setup in
this virtual environment (e.g., a delayed reward
task, an exploration paradigm) and connect the
computational model to this paradigm. As for
usual computational modeling, the chosen biolog-
ical measurements (e.g. neural activity, task suc-
cess performance) are simulated when running the
model. Such model is indeed expected to repro-
duce qualitatively the ground truth, for a given set
of parameters value. A step further, it is very im-
portant to numerically verify what happens when
modifying any quantitative or qualitative parame-
ter value. If the numerical sensibility is so strong
that the results cannot be reproduced for some tiny
parameter variation, the model is meaningless be-
cause biological values make sense as a numerical
range, not a single number. If the numerical sen-
sibility is so weak that any parameter value pro-
duces the expected result, this parameter is mean-
ingless and a simpler model very likely explains
the same data set. The key-point here is that such
usual model predictive verification is not only go-
ing to be possible, given a fixed data set, but for
any data set obtained running experiment in the
virtual environment. In other words, the computa-
tional model variants are going to be always tested
in-situ. With no practical bounds on the experi-
mental variants (e.g., number of trials, sensory in-
NEUROTECHNIX2014-InternationalCongressonNeurotechnology,ElectronicsandInformatics
158

Figure 3: The software’s graphic user interface. In order to observe and control the experiment, a view of the bot vision,
status, input and output is available via interactive panels. It is also possible to “cheat” by controlling these variables values,
in order to debug an experiment and understand in details what happens in such a complex system interaction.
put precision, task complexity).
• Design new experimental paradigms to confront
the computational model to falsifiable conditions :
Building on an animal model an experiment,
training the animals for weeks, restarting from
the beginning, if it appears that there is an unex-
pected trap (e.g., the task in too simple or unfeasi-
ble, or does not allow to discriminate between two
concurrent models) may be a huge work. Start-
ing to design the experiment in a virtual envi-
ronment completely changes the method: the hy-
pothesized computational model is first tested in
silico (i.e., neither in vivo, nor in vitro, but in a
software environment) and only confronted to the
biological reality in a second stage. The work-
plan is inverted with respect to usual neuroscience
studies, but in computational engineering (e.g.,
designing new airplanes) this is exactly the way
it goes until a few decades. It is however not
new in neuroscience, at the scale of mesoscopic
brain map study (see e.g., (Chemla et al., 2007) or
(Brette et al., 2007)). The key point here, is that
such approach is now possible at the behavioral
level, considering sensory-motor interactions with
a simple or complex environment. Such process
also obviously yields a parsimonious use of ani-
mal models. A step further, it lays down the chal-
lenge to perform realistic experiment with a com-
putational model of the brain behavior, not only
consider toy situations where the plausibility of
the model can not be checked.
The degree of equivalence between simulation
outcome and neuroscience experimental results is key
issue. This is the reason why we have chosen a soft-
ware platform where usual systemic neuroscience ex-
periments can be reproduced “as a whole”, as illus-
trated by several examples throughout this draft. Ba-
sis omissions (e.g., level of modeling detail) have
been justified or rejected, in order to attain this objec-
tive of simulating classes of behavioral experiments
where the subject is trained to realize a survival or
rewarding task in a known or unexpected environ-
ment. Applications include Pavlovian (e.g., action
of the amygdala) or operant conditioning, reinforce-
ment learning, training and habituation, task oriented
focus of attention, multi-sensory interaction. The
main brain structures involved in such tasks are the
basal ganglia system (including afferent and efferent
structures) regarding selection of action, the different
memory structures (e.g., episodic memory in the hip-
pocampus, or working memory in the prefrontal cor-
tex).
5 DISCUSSION
Beyond these basic features, the input and output can
be easily manipulated in order to enlarge the exper-
imental setup. Up to now, the main aspect is “input
or output degradation”, i.e. adding noise. Originally,
bot perception and action are performed without any
added noise or random mistake. Depending on the ex-
VirtualEnaction-APlatformforSystemicNeuroscienceSimulation
159

periment to be conducted, it is obvious (i.e. inserting
a few lines of code between the platform and the bot),
either to reduce variables precision range (e.g., add
noise to the pixelic image) or to randomly draw the
fact that a gesture may succeed or fail (e.g., introduce
spurious command).
A step further, as already implemented as plug-
ins, since all environment elements are available (not
to the bot, but to the experiment software), it would
be possible to design other cues, or more generally
other interactions with the environment. However, in
collaboration with neuroscience experimentalists, we
have carefully selected what seems useful to explore
biological systemic models, and avoided to provide a
too general tool that do anything.
Building one “brainy-bot” is a rather huge task
and requires several high-level cognitive functional-
ity. However, though systemic neuroscience requires
to study the system as a whole, it does not imply that
each functionality has to be studied at the same level
of details. Several blocks may be considered as black-
boxes interacting with the part of the system to be ex-
tensively studied. This is the reason why the present
platform is not limited to a survival environment, but
comes also with middle-ware (presently in develop-
ment) related to the basic cognitive functionality in-
volved in such paradigms, as listed in Appendix 5.
Some modules will thus be implemented according
to a rough description, e.g., via an algorithmic ersatz.
The nervous sub-system under study, on the reverse is
going to be implemented at a very fine scale (neural
network mesoscopic models or even spiking neural
networks).
The key features of this digital experimenta-
tion platform include the capability to perform ex-
periments involving both long-term continuous time
paradigms or short-term decision tasks with a few
time-steps. It also allows us to consider either sym-
bolic motor command or sensory input (e.g., ingest
or not food, detect the presence of a stimulus) or
quantitative gestures and complex trajectory genera-
tion (e.g., find resources in an unknown environment).
A key point is to be able to mimic and repeat at will
experiments performed in neuroscience laboratory on
animals. Here, the obtained computational models are
not only going to “fit the data” but to be explored far
beyond, yielding the possibility to study long-term
adaptation, statistical robustness, etc. Not only one
instance of a bot can be checked, but several parallel
experiments can be run in order to explore different
parameter ranges, or compare alternative models.
It would also be instructive to better understand to
which extent such bio-inspired architectures actually
required to control a biological system could enhance
artificial control rules commonly applied in robotics
or game engines. This is a challenging issue, beyond
the present study, but an interesting perspective of the
present work.
As a conclusion, let us mention that this platform
has already been used for preliminary digital exper-
iments about Pavlovian conditioning (Gorojosky and
Alexandre, 2013) involving the functional modeling
of the amygdala and hippocampus, decision making
mechanisms in link with reinforcing signals yielded
by aversive or appetitive stimuli and internal com-
putation (Beati et al., 2013), plus a student work of
the AGREL connexionist categorization model here
confronted to a realistic environment (Carrere and
Alexandre, 2013).
ACKNOWLEDGMENTS
This work was partly supported by the KEOpS ANR
project. Huge thanks to Nicolas Rougier for precious
advises and Maxime Carrere for his feedback. The
NeBICA’14 review was a real chance to improve the
original draft, thanks.
REFERENCES
Beati, T., Carrere, M., and Alexandre, F. (2013). Which re-
inforcing signals in autonomous systems? In Third In-
ternational Symposium on Biology of Decision Mak-
ing, Paris, France.
Brette, R., Rudolph, M., Carnevale, T., Hines, M., Beeman,
D., Bower, J. M., Diesmann, M., Morrison, A., Good-
man, P. H., Harris, F. C., Zirpe, M., Natschl
¨
ager, T.,
Pecevski, D., Ermentrout, B., Djurfeldt, M., Lansner,
A., Rochel, O., Vieville, T., Muller, E., Davison, A. P.,
El Boustani, S., and Destexhe, A. (2007). Simulation
of networks of spiking neurons: a review of tools and
strategies. Journal of computational neuroscience,
23(3):349–398.
Carrere, M. and Alexandre, F. (2013).
´
Emergence
de cat
´
egories par interaction entre syst
`
emes
d’apprentissage. In Preux, P. and Tommasi, M.,
editors, Conf
´
erence Francophone sur l’Apprentissage
Automatique (CAP), Lille, France.
Chemla, S., Chavane, F., Vieville, T., and Kornprobst, P.
(2007). Biophysical cortical column model for optical
signal analysis. BMC Neuroscience, 8(Suppl 2):P140.
Cofer, D., Cymbalyuk, G., Reid, J., Zhu, Y., Heitler, W. J.,
and Edwards, D. H. (2010). AnimatLab: a 3D
graphics environment for neuromechanical simula-
tions. Journal of neuroscience methods, 187(2):280–
288.
Davison, A. P., Br
¨
uderle, D., Eppler, J., Kremkow, J.,
Muller, E., Pecevski, D., Perrinet, L., and Yger, P.
(2008). PyNN: A Common Interface for Neuronal
NEUROTECHNIX2014-InternationalCongressonNeurotechnology,ElectronicsandInformatics
160

Network Simulators. Frontiers in neuroinformatics,
2.
Denoyelle, N. and Pouget, F. (2014). Virtualenaction,
user documentation. Technical report, virtualenac-
tion.gforge.inria.fr.
Friston, K. (2012). A Free Energy Principle for Biological
Systems. Entropy, 14(11):2100–2121.
Gorojosky, R. and Alexandre, F. (2013). Models of
Hippocampus for pavlovian learning. Rapport de
recherche RR-8377, INRIA.
Hyv
¨
arinen, A. (2009). Natural image statistics a probabilis-
tic approach to early computational vision. Hardcover.
Pouget, F. and Denoyelle, N. (2014). Virtualenaction, de-
veloper documentation. Technical report, virtualenac-
tion.gforge.inria.fr.
Taouali, W., Vi
´
eville, T., Rougier, N. P., and Alexandre, F.
(2011). No clock to rule them all. Journal of physiol-
ogy, Paris, 105(1-3):83–90.
Teftef, E., Escobar, M.-J., Astudillo, A., Carvajal, C., Ces-
sac, B., Palacios, A., Vi
´
eville, T., and Alexandre, F.
(2013). Modeling non-standard retinal in/out function
using computer vision variational methods. Rapport
de recherche RR-8217, INRIA.
Uithol, S., van Rooij, I., Bekkering, H., and Haselager, P.
(2012). Hierarchies in action and motor control. Jour-
nal of cognitive neuroscience, 24(5):1077–1086.
Vi
´
eville, T. and Crahay, S. (2004). Using an Hebbian learn-
ing rule for multi-class SVM classifiers. Journal of
Computational Neuroscience.
Vi
´
eville, T. and Vadot, C. (2006). An improved biologically
plausible trajectory generator. Technical Report 4539-
2, INRIA.
APPENDIX
A BRAINY-BOT GENERIC
FUNCTIONALITY
Even when restraining to functional modeling, it is
not possible to simulate all sub-systems of the brain
at the same level of details. In order to help repre-
senting the part of the system that may be simulated
without biologically plausible models in a given con-
text, a few sets of functionality are proposed. Let us
briefly present the main algorithmic cues.
- Episodic memory of input/output: Any system has
not only to take into account the present input and
output in order to generate a proper behavior, but also
store and consider the recent past information. In the
brain, this function is mainly located in the hippocam-
pus. In our context, thanks to the design choices, such
information corresponds to a simple file of symbolic
information, namely a hierarchical itemization of pa-
rameter values (i.e. a XML data structure). As a con-
sequence, the memorization, transmission, compres-
sion (in the sense of eliminating negligible values or
values related to older or smaller elements) is easy
to well-define. It has also the consequence to pro-
pose a generic internal representation of the sensory-
motor information at a symbolic level, without both-
ering about how this information is encoded on neural
maps. It is an interesting perspective of the present
work and an ongoing work to further investigate this
issue, while basic algorithmic modules for such data
management are already available.
- Trajectory generation: At a functional level a “be-
havior” (i.e., a complex gesture) can be specified as
finding a path from an initial state (e.g., being hun-
gry while food is known to be present elsewhere)
to a final state (e.g., having the food ingested) tak-
ing constraints into account (e.g., avoiding or mov-
ing aside obstacles on the way). Such issue may be
a topic, or not (e.g., when studying the selection of
action we may prefer not to bother with the planifica-
tion and execution of such actions). In the latter case,
generic specification of such problem and universal
algorithms to solve them exist, in relation with har-
monic control which is a biologically plausible frame-
work (i.e., with fully distributed computation based
on diffusion mechanism) (Vi
´
eville and Vadot, 2006).
- Generic categorization: Another generic key cogni-
tive feature is the capability to “extract” symbolic in-
formation from a bundle of quantitative or qualitative
values. This includes sensory events detection (e.g.,
detect the presence of predator from sensory cues),
object labeling (e.g., an element as a resource to in-
gest). Though such issue is indeed a topic on its own,
we may wish in some context to have it available as
a black box. A biologically plausible support vector
machine mechanism is available to this end, with ver-
satile uses (Vi
´
eville and Crahay, 2004). Let us also
mention that informing the bot about its absolute po-
sition and orientation or about symbolic information
of the scene is a way to shortcut its sensory modules
and provide integrated cognitive information.
B COMPUTER
IMPLEMENTATION
This experimental virtualenaction setup is fully
documented both at the user (Denoyelle and Pouget,
2014) and developer (Pouget and Denoyelle, 2014)
levels. The user view of the computer implemen-
tation is oversimple as illustrated in Fig. 4, though
the underlying computer development is not that triv-
ial as sketched out in Fig. 5. It runs a bukkit
server in the 1.4.5 version and is designed as a set
of plugin for this server, with minimal changes in
the original code. This design choice was optimal
VirtualEnaction-APlatformforSystemicNeuroscienceSimulation
161
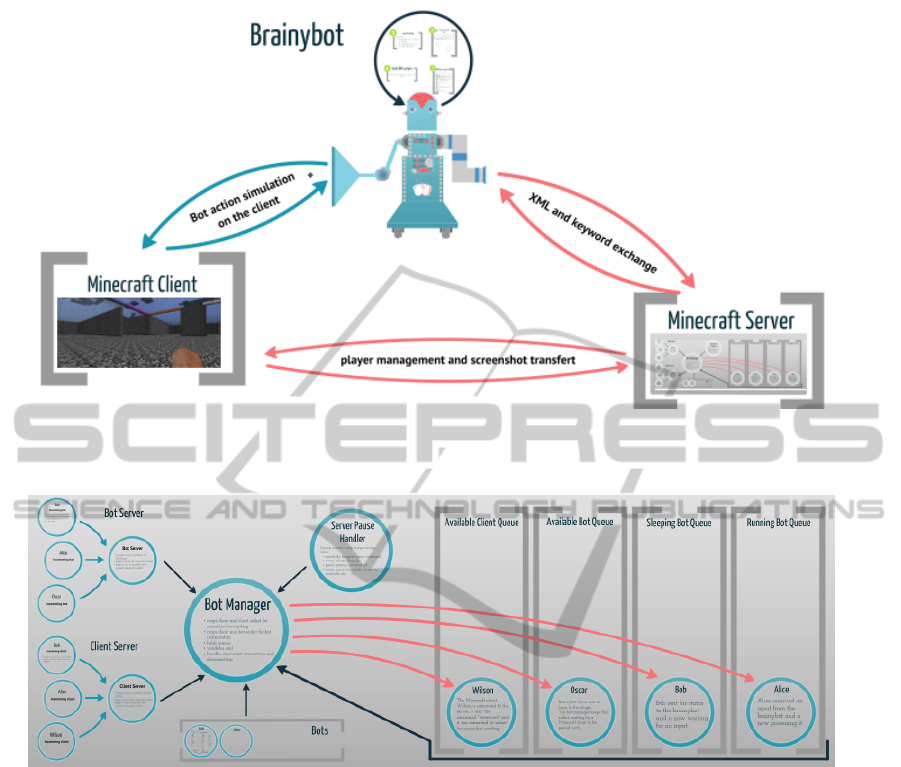
Figure 4: The platform’s architecture. At the user level, only the notion of (i) game server where the environment and its
mobile objects interactions are computed, (ii) game client where the game-play is rendered and (iii) brainy-bot where the
brain model simulation is issued have to be taken into account.
Figure 5: The platform underlying protocol. At the system level, the synchronization between the existing game events and
the different system component is a non trivial middle-ware, taking into account both “real players” (thus playing in real time)
and “bot players” (with slow reaction delays). The developed middle-ware can cope with several bots and humans (which
will have to be patient, since the game runs at a reduced rate). Furthermore, the design allows the platform to be distributed
on a cluster (server, client and bots running on different machines).
in terms of middle-ware development effort and fi-
nal code stability. At the server level, two plugins
have been designed. The VirtualEnaction plugin
allows us to replace usual player mouse and keyboard
interactions with the game-play by a programmable
API. The Characteristics plugin allows us to im-
plement blocks and entities additional characteristics
such as temperature, hardness or smell.
The minecraft server and client have been modi-
fied in order to be able to run in this programmatic
mode, and to get a full access to all game gauges, be-
cause we not only must run the bot but also observe in
details its behavior and measure all interactions with
the environment. As a consequence, the actual source
code corresponds to a fork of a frozen version of the
minecraft game, while everything is documented in
order to easily migrate to some more recent version.
Another key point is the modification of the en-
vironment. Using the standard game, it is possible
to build an environment, include other entities, and
so on. This is simply realized via the game interface.
Futhermore, scripted scenarios can also be introduced
(e.g., some resource appears if and only if a block is
put in a given position), as additional plugins. In ad-
dition, configuration files on the server side, allows us
to act on available resources or survival gauges, and
build versatile survival situations. It is thus a fully
editable setup. As being an open-source collaborative
software, it is available on the Inriaforge with more
than 15 co-developers or project followers.
A step further, in order this virtual experimenta-
tion platform to be extensible in the perspective of
NEUROTECHNIX2014-InternationalCongressonNeurotechnology,ElectronicsandInformatics
162

new setups, the botplug that allows the bot to be
plugged in the client/server system is also designed
with a notion of plugin. For instance, a tactile sense
(not described here), and a variant of the pixelic vision
to be connected to different image sequence compu-
tation libraries are made available as plugins.
It is clear that several extensions are going to be
developed in a near future thanks to this modular ar-
chitecture choice.
VirtualEnaction-APlatformforSystemicNeuroscienceSimulation
163
