
Exploring Neural Principles with Si elegans, a Neuromimetic
Representation of the Nematode Caenorhabditis elegans
Axel Blau
1
, Frank Callaly
5
, Seamus Cawley
5
, Aedan Coffey
5
, Alessandro De Mauro
4
, Gorka Epelde
4
,
Lorenzo Ferrara
1
, Finn Krewer
5
, Carlo Liberale
1
, Pedro Machado
2,3
, Gregory Maclair
4
,
Thomas Martin McGinnity
2,3
, Fearghal Morgan
5
, Andoni Mujika
4
, Alexey Petrushin
1
,
Gautier Robin
4
and John Wade
2,3
1
Dept. of Neuroscience and Brain Technologies (NBT) and Nanostructures Unit (NAST),
Fondazione Istituto Italiano di Tecnologia (IIT), 16163 Genoa, Italy
2
Intelligent Systems Research Centre (ISRC), University of Ulster, Derry BT487JL, Londonderry, Northern Ireland
3
School of Science and Technology, Nottingham Trent University, Nottingham, U.K.
4
eHealth and Biomedical Applications (eHBA), Vicomtech-IK4, San Sebastián, Spain
5
Bio-Inspired Electronics and Reconfigurable Computing (BIRC), National University of Ireland, Galway, Ireland
Keywords: Brain-Inspired Computation, Nervous System Emulation, Soft Body Simulation, Virtual Embodiment,
Neurocomputational Response Models on Field-Programmable Gate Arrays (FPGAs).
Abstract: Biological neural systems are powerful, robust and highly adaptive computational entities that outperform
conventional computers in almost all aspects of sensory-motor integration. Despite dramatic progress in
information technology, there is a big performance discrepancy between artificial computational systems
and brains in seemingly simple orientation and navigation tasks. In fact, no system exists that can faithfully
reproduce the rich behavioural repertoire of the tiny worm Caenorhabditis elegans which features one of the
simplest nervous systems in nature made of 302 neurons and about 8000 connections. The Si elegans project
aims at providing this missing link. This article is sketching out the main platform components.
1 INTRODUCTION
Caenorhabditis elegans, a soil-dwelling worm with
a life span of a few days, 1 mm long and 80 µm in
diameter, is one of the five best characterized
organisms. It is multicellular and develops from a
fertilized egg to an adult worm like any other
animal. Although its genome is small (~ 10 M base
pairs), there is about 40% homology to the human
genome (3.2 G base pairs). The adult hermaphrodite
is comprised of exactly 959 cells, including 95 body
wall muscle cells and 302 neurons. Despite its
simplicity, the nervous system of C. elegans does
not only sustain vital body function, but generates a
rich variety of behavioural patterns in response to
internal and external stimuli. These include
associative and several forms of nonassociative
learning that persist over several hours (Hobert,
2003). Interestingly, many processes of learning and
memory in C. elegans are highly conserved across
evolution, which suggests that there are universal
information processing mechanisms throughout the
animal kingdom (Lin and Rankin, 2010). With all of
this data, information and modern computer
technology at hand, it is surprising that there is yet
no comprehensive artificial C. elegans emulation
system from which the principles of neural
information processing underlying behaviour can be
derived. The Si elegans project aims to fill this gap.
It will do so by implementing completely
reconfigurable neuronal models on FPGA modules
representing individual neurons. Signals between
neurons within the neural circuitry will be
exchanged by light. Such optical free-space
interconnection concept promises to be one of the
most attractive solutions for overcoming the
‘interconnectivity crisis’, currently one of the most
serious bottlenecks in upscaling neuromorphic
network architectures. In a 3D configuration, a
quasi-limitless number of connections between
modules can be established due to the fact that light
beams do not interfere with each other. It
furthermore allows the easy reconfiguration of
189
Blau A., Callaly F., Cawley S., Coffey A., de Mauro A., Epelde G., Ferrara L., Krewer F., Liberale C., Machado P., Maclair G., Martin McGinnity T., Morgan
F., Mujika A., Petrushin A., Robin G. and Wade J..
Exploring Neural Principles with Si elegans, a Neuromimetic Representation of the Nematode Caenorhabditis elegans.
DOI: 10.5220/0005190701890194
In Proceedings of the 2nd International Congress on Neurotechnology, Electronics and Informatics (-2014), pages 189-194
ISBN:
Copyright
c
2014 SCITEPRESS (Science and Technology Publications, Lda.)

network connectivity by simply exchanging (or in
case of active optical elements by reprogramming)
the light distribution elements. Finally, due to the
inherent scalability of the approach, more complex
networks can be emulated.
2 THE Si elegans APPROACH
We currently develop a hardware-based computing
framework that will accurately mimic C. elegans in
real time and enable complex and realistic behaviour
to emerge through interaction with a rich, dynamic
simulation of a natural or laboratory environment.
We initiated to replicate the nervous system of C.
elegans on a highly parallel, modular, user-
programmable, reconfigurable and scalable FPGA
hardware architecture. It will be embodied in a
virtual environment for behavioural studies. The
virtualization will take the sensory-motor loop and
realistic body physics into account. The resulting
computational platform will be provided through an
open-access web portal to the scientific community
for its peer-validation and use (Figure 1).
Figure 1: The three main elements of the Si elegans
platform: 1. the hardware emulation of the C. elegans
nervous system, which is 2. virtually embodied and
interacting with an artificial environment that can 3. be
defined by users.
Several innovative key concepts will ensure the
accurate mimicry of the C. elegans nervous system
architecture and function. Each of the 302 neurons
will be represented by individual field-
programmable gate array (FPGA) modules, each of
them being independently and dynamically
programmable with a user-specific and parame-
terised neuronal response model through a user-
friendly neuron model submission and configuration
facility or through selection from a library of pre-
defined and tested neuron models. Pioneering
electro-optical interconnection schemes will allow
dense module distribution and parallel, interference-
free inter-neuron communication in a 3D space. In a
closed-loop feedback design, this hardware blueprint
of the C. elegans nervous system will control a
biophysically correct virtual representation of the
nematode body in a virtual behavioural setting.
Instead of limiting its function and impact on
science and technology by imposing pre-made
models only, the Si elegans framework will be made
available to the worldwide scientific community
through an open-access web-portal. It will feature an
intuitive and user-friendly remote configuration
interface to define an unlimited number of neuron
models and information processing hypotheses for
automatic FPGA hardware configuration. This peer-
participation concept will not only warrant the
independent and unbiased functional validation of Si
elegans, but permit the iterative optimization of
neuron models and the asymptotical approach
towards a holistic reproduction and understanding of
the complete set of C. elegans behaviours and their
underlying nervous system mechanisms through a
set of reverse-engineering tools.
Two core aspects govern the project. The first
addresses the technological design and assembly of
the Si elegans hardware architecture accompanied
by the development of the virtual arena and of the
neural response model design and FPGA
configuration interface followed by their integration
into a user-friendly web-accessible platform. The
second addresses its deployment to the scientific
community for its independent peer-validation as a
free-access tool and testbed for neurocomputational
studies.
The hardware design includes individual neuron
modules, each consisting of three elements in their
simplest embodiment as sketched out in Figure 2: i)
a synaptic/gap junction input array, ii) postsynaptic
processing of synaptic/gap junction input based on
the selected neuron-specific model that has been
implemented on dynamically (re)programmable
FPGA circuitry for the arbitrary definition of the
type of neuron and its response behaviour, and iii)
an axonal output line that distributes the neural
response (e.g., spikes, membrane fluctuations)
simultaneously to individual synapses of one or
many target neurons.
In a coarse comparison, i) represents individual
synapses/gap junctions, ii) the soma and dendritic
tree of a neuron, and iii) the axon with its axonal
arbour. The spatial organization and assembly of the
Si elegans nervous system will be based on the
NEUROTECHNIX2014-InternationalCongressonNeurotechnology,ElectronicsandInformatics
190

published connectome (taking most recent and new
findings or hypotheses published during project time
into account (e.g., (G. Haspel and O'Donovan,
2011)).
Figure 2: Concept and elements of an individual Si
elegans FPGA neuron module and its comparison with a
real neuron. Neural activity will arrive at individual input
lines of an FPGA (i) and will be processed by the neuron-
specific stimulus-response algorithm that the FPGA was
programmed with (ii). Its output activity will be
distributed in parallel through signal distribution elements
(iii) to individual input lines (i) of the target FPGA
neurons to which the neuron connects to. In case of signal
propagation by light, incoming activity will arrive as
spatially confined light pulses at individual pixels of
photoelectric converters (synapses/gap junctions) being
individually connected to the individual FPGA input lines.
Neural response activity generated by the neural model
residing on the FPGA will trigger a coherent light source
at one of its output lines (axon). This light will pass
through microoptical light-structuring elements to
distribute activity onto selected pixels (synapses/gap
junctions) of interconnected target neurons. In case of
electrical signal transmission through wires, a split-wire
bundle will transmit a digital signal pulse from the axonal
output line of the FPGA to individual synapse/gap
junction sockets of the target neurons.
2.1 FPGA Representations of
C. elegans Neurons and Muscles
The Si elegans emulation framework is composed of
distinct and independent modular components. To
date, the majority of hardware-assisted simulation
efforts have focused on mirroring a complete
network of several neurons on a single FPGA chip
or in ASIC/VLSI technology. Si elegans, in contrast,
aims at dedicating a single FPGA module to a single
neuron to emulate neuron-specific stimulus-response
models at quasi arbitrary resolution. While from an
engineering point of view this seems to be a waste of
hardware resources at first glance, this approach will
give room for implementing neural processing
schemes of arbitrary complexity allowing for the
consideration of all types of intracellular events
(e.g., signalling cascades etc.). This will allow
biologists and computational neuroscientists to
extend model complexity and fidelity to unimagined
high degrees.
By means of dynamically reconfigurable field
programmable gate arrays (drFPGAs) the inherent
signal processing and response logic of each neuron
will be reprogrammable. The need for dynamically
reprogrammable somato-dendritic circuitry has
several advantages: to stay flexible in defining the
type and thus the response behaviour of a neuron, to
implement any kind of synaptic or dendritic pre-
processing algorithms, to freely adjust or upgrade
the algorithms for emulating neural development
(changes in neural response) or implementing
upcoming neuroscience knowledge, and to emulate
disease states (e.g., Parkinson's, epileptic seizures)
by temporarily modulating the response behaviour at
run-time. drFPGAs thus offer the best ratio between
hardware costs and performance, accuracy, and
parameterization space. Since FPGA technology
currently experiences fast technological advances, it
will also be easy to exchange individual modules for
more powerful or smaller ones at any time.
2.2 3D Interconnection of FPGA
Modules to Replicate the
C. elegans Connectome
In almost all hardware implementations of neural
networks, the issue of inter-neuron connectivity is a
major problem. Serial-type simulations introduce
stochastic jitter in the timing of events and thus fail
to accurately and reproducibly mimic parallel
information flow between neurons. If a parallel
inter-neuron connectivity is implemented on-chip
instead (e.g., by using ASIC technology), typically
90% of the chip is composed of interconnect and
scaling networks becomes a major problem. In this
project we are proposing to solve this problem by
using optical or wire-based off-chip interconnects.
Two complementary interconnection strategies will
be pursued and compared for their ease of
ExploringNeuralPrincipleswithSielegans,aNeuromimeticRepresentationoftheNematodeCaenorhabditiselegans
191

implementation, reliability, functionality and
scalability. They will be implemented by adding two
elements to each FPGA neuron module: i) a
synaptic/gap junction input field (pixel matrix for
wire connection, optical fibre plugs or light-
receptive pixels with light-to-charge conversion) and
an axonal output line with distribution elements that
communicate the neural response simultaneously to
individual synapses of one or many target neurons.
For the optical information transmission among
neurons, fast-switchable and intensity tuneable laser-
diodes are the emission light source(s) of choice.
They are triggered by the axonal output pin of an
FPGA neuron module. Their light is structured and
thus projected onto individual synaptic or gap
junction inputs of those target neurons that the active
neuron connects to. Any combination of reflective,
refractive, diffractive and masking elements such as
mirrors, micromirror arrays, microprism or
microlens arrays, gratings, colour filters and etched
shadow masks will be explored to ensure the correct
addressing and optical information transfer between
sending and receiving FPGA neurons. Synapses and
gap junctions of the receiving neurons will be
represented by arrays of photoelectric elements.
2.3 A Virtual Arena for Behavioural
Studies
Several C. elegans-specific descriptors of its
physiology, morphology and body mechanics exist,
including a realistic representation of the body (e.g.,
Virtual Worm Project, neuroConstruct) and aspects
of locomotion (Boyle, 2009; Bryden and Cohen,
2008; Gal Haspel, O'Donovan, and Hart, 2010;
Mailler, Avery, Graves, and Willy, 2010; Niebur and
Erdos, 1993; Stephens, Johnson-Kerner, Bialek, and
Ryu, 2010; Wakabayashi, 2006). Based on these
data, the Si elegans hardware nervous system of C.
elegans will be embodied through a biophysically
realistic virtual representation of the nematode in a
virtual environment. The virtual body will share the
shape, body-physics (e.g., elasticity, friction) and
cellular organization of C. elegans (including
realistic spatio-functional representations of sensory
cells). The interplay between active actuation
through sensory-driven control circuits of its
nervous system and passive actuation by
environmental factors (material-properties, arena
topology, gravity, air- or fluid-flow etc.) will be
considered. The simulation of this virtual body being
situated in a virtual arena will be running on
standard PC hardware. The virtual arena can be
freely configured to copy the 3D geometries and
biophysical features of an experimental environment
used in in vivo studies. It displays the native
behaviour of the C. elegans representation, provides
simulated environmental stimuli to its sensory
neurons and shows stimuli-induced responses (e.g.,
muscle actuation, secretory events). Information
flow is channelled in real-time through a bi-
directional interface between the computer and the
Si elegans nervous system. Sensory neurons of the Si
elegans nervous system receive their input from
programmable light sources. Si elegans’ motor
neuron activity actuates associated muscles of the
virtual body. Through a real-time closed-loop
feedback, any resulting sensory experience (e.g.,
change in posture, touch, change of chemical
concentration gradients) is coded and transmitted to
the Si elegans nervous system emulation as new
sensory input.
2.4 Neural Model Definition User
Interface and Network State
Analysis
The modelling space shall allow for the definition of
relevant neural processing parameters in a pictorial,
object-oriented flow diagram (graphical drag-and-
drop manner) or script. The desired network
structure can be created by simply selecting various
neuron, synapse or gap junction models from a
library of available components and connecting
them together. Alternatively, existing neural
response models can be imported from other
simulation engines (e.g., NEURON, BRIAN,
NeuroML, ...) (Figure 3).
An assembly can be stored as a neuron-specific
model to become a high-level, properly documented
building block for other researchers. The modelling
toolset provides all required design elements to
address and freely combine all known features and
events of neural signal transmission down to the
synaptic level, possibly including even abstracted
models of signalling cascades. These design
elements can be altered, thus personalized and
versioned by community members for experimental
purposes. E.g., the function of an individual synapse
may include cable properties of the pre-synaptic
axon and synapse to account for physiological and
morphological boundary conditions that shape/affect
signal properties such as signal transmission delays
and attenuation. We furthermore implement a
standard set of amplitude-invariant, self-terminating
action potentials with stereotyped waveforms as well
as graded regenerative potentials as the predominant
signal type in C. elegans with amplitudes and
NEUROTECHNIX2014-InternationalCongressonNeurotechnology,ElectronicsandInformatics
192
waveforms that are highly sensitive to the size,
duration and waveform of the stimulus. Finally, the
Si elegans platform will provide readout and storage
tools for reverse-engineering nervous-system
function.
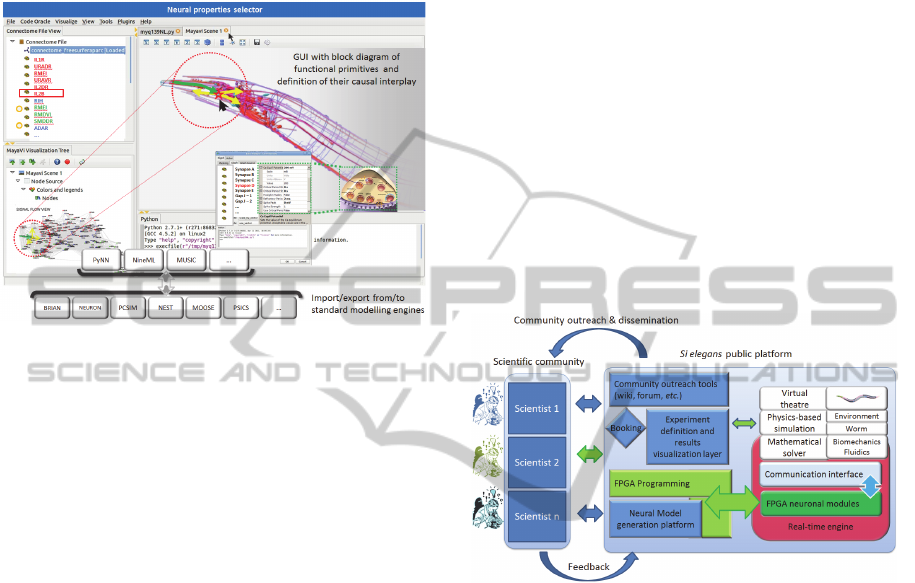
Figure 3: Si elegans GUI mock-up for model definition.
We will provide users with a flexible GUI for the user-
friendly and intuitive definition of neuron-specific
response models for the efficient and fast design-to-FPGA
prototyping of neural models. It will provide ready-made
neuron models and sets of parameterisable components or
functional primitives from which scientists can construct
their own neural response models. The definition
environment will contain a virtual representation of C.
elegans including the locations and connections of
individual neurons and muscle cells (and everything else).
The user may zoom into the nervous system and click on a
neuron, which will then show its incoming and outgoing
connections within the nervous system along with their
morphologically correct pathways from the source neurons
(incoming) towards the target neurons/cells (outgoing).
Upon double-click of elements in the GUI a dialog will
open for defining the properties of individual input-output
algorithms (neural response model - e.g., ion-channel
response, synaptic integration etc.). A CAD-program-like
tree lists all features/synapses/primitives of the particular
neuron. A separate tab allows the definition of information
flow among individual elements (blocks) within a
particular neuron (e.g., dendro-somatic kinetics, leakage
…). Alternatively, these parameters may also be imported
from other simulation engines or be defined by a
Python/PyNN definition script.
2.5 Deployment of the Si elegans
Platform for Public Access and
Peer-Contribution
Given that there are over 300 laboratories worldwide
working on C. elegans, a majority of them studying
some aspect of nervous system function, there is
considerable interest in modelling it. The seemingly
trivial complexity of the nematode C. elegans has
kept researches busy over the past 50 years without
revealing a complete understanding of the
functioning of its nervous system. The spirit and
central mission of Si elegans as an ‘open-science,
peer-contribution’ project is therefore the early
involvement of the scientific community,
particularly groups interested in behavioural and
modelling aspects of C. elegans, but also the neuro-
computation community at large. The Si elegans
platform will therefore be made accessible to the
scientific community (e.g., in a shared-time, fair-use
model) through a client/server-based remote access
environment not only for the pursuit of scientific
studies, but also for its independent validation and
enhancement through extensions (e.g., through plug-
ins). Feedback from researchers will be shared
through community outreach tools and be
implemented into the public platform (Figure 4).
Figure 4: Schematic overview on the Si elegans platform
elements and their accessibility by the scientific
community.
3 CONCLUSIONS
While Si elegans restricts itself to the emulation of
the C. elegans nervous system, the underlying
design concepts have universal application. Si
elegans will constitute a generalizable framework
from which the universal working principles of
nervous system function can be induced, and new
scientific knowledge on higher brain function and
behaviour can be generated. By being designed for
open-source and peer-contribution, Si elegans
promises to become a generalizable testbed for
neurobiological signal processing hypotheses in
healthy and disease-affected nervous systems in
neuroscience, neurocomputation and neurology. It
may also lay the foundation for exploring and
refining new neuromimetic computational concepts
ExploringNeuralPrincipleswithSielegans,aNeuromimeticRepresentationoftheNematodeCaenorhabditiselegans
193

to provide a blueprint for the design of biologically
inspired, brain-like parallel processing hardware
architectures that are orthogonal to current von
Neumann-type machines. If a holistic and verifiable
understanding of C. elegans nervous system function
could be achieved, a long-locked door would open to
implement such knowledge in neuromimetic
processing and control mechanisms in any
technological field, be it robotics, medical
assistance, decision-making devices, fraud-detection
or surveillance.
ACKNOWLEDGEMENTS
The Si elegans project 601215 is funded by the 7
th
Framework Programme (FP7) of the European
Union under FET Proactive, call ICT-2011.9.11:
Neuro-Bio-Inspired Systems (NBIS).
REFERENCES
Boyle, J. H. (2009). C. elegans locomotion: an integrated
approach. (PhD Thesis), University of Leeds, Unitied
Kingdom. Retrieved from http://etheses.whiterose.
ac.uk/1377/
Bryden, J., and Cohen, N. (2008). Neural control of
Caenorhabditis elegans forward locomotion: the role
of sensory feedback. Biological Cybernetics, 98(4),
339-351. doi: 10.1007/s00422-008-0212-6
Haspel, G., and O'Donovan, M. J. (2011). A perimotor
framework reveals functional segmentation in the
motoneuronal network controlling locomotion in
Caenorhabditis elegans. Journal of Neuroscience,
31(41), 14611-14623. doi: 10.1523/JNEUROSCI.
2186-11.2011
Haspel, G., O'Donovan, M. J., and Hart, A. C. (2010).
Motoneurons Dedicated to Either Forward or
Backward Locomotion in the Nematode Caenor-
habditis elegans. The Journal of Neuroscience, 30(33),
11151-11156. doi: 10.1523/jneurosci.2244-10.2010
Hobert, O. (2003). Behavioral plasticity in C. elegans:
Paradigms, circuits, genes. Journal of Neurobiology,
54(1), 203-223. doi: 10.1002/neu.10168
Lin, C. H., and Rankin, C. H. (2010). Nematode Learning
and Memory: Neuroethology. In M. D. Breed and J.
Moore (Eds.), Encyclopedia of Animal Behavior (pp.
520-526). Oxford: Academic Press.
Mailler, R., Avery, J., Graves, J., and Willy, N. (2010). A
Biologically Accurate 3D Model of the Locomotion of
Caenorhabditis Elegans. Paper presented at the 2010
International Conference on Biosciences
(BIOSCIENCESWORLD).
Niebur, E., and Erdos, P. (1993). Theory of the
locomotion of nematodes: control of the somatic
motor neurons by interneurons. Mathematical
Biosciences, 118(1), 51-82.
Stephens, G. J., Johnson-Kerner, B., Bialek, W., and Ryu,
W. S. (2010). From Modes to Movement in the
Behavior of Caenorhabditis elegans. PloS One, 5(11),
e13914. doi: 10.1371/journal.pone.0013914
Wakabayashi, M. (2006). Computational Plausibility of
Stretch Receptors as the Basis for Motor Control in C.
elegans. (BA Thesis), Univ. of Queensland, Australia.
Retrieved from http://archive.itee.uq.edu.au/~markw/
celegans/thesis.pdf?origin=publication_detail
NEUROTECHNIX2014-InternationalCongressonNeurotechnology,ElectronicsandInformatics
194
