Transcutaneous Spinal Direct Current Stimulation
Modelling the Electric Field Distribution in the Cervical Spinal Cord
S. R. Fernandes
1,2
, R. Salvador
1
, C. Wenger
1
, M. de Carvalho
2
and P. C. Miranda
1
1
Institute of Biophysics and Biomedical Engineering (IBEB), Faculdade de Ciências,
Universidade de Lisboa, 1749-016 Lisboa, Portugal
2
Molecular Medicine Institute (IMM), Faculdade de Medicina, Universidade de Lisboa,
Avenida Professor Egas Moniz, 1649-028, Lisboa, Portugal
1 OBJECTIVES
Following the reports that weak electrical currents can
modulate excitability in cortical areas, Cogiamanian et
al. (2012) proposed that the same approach could be
applied to modulate spinal cord function. Exploratory
studies in humans showed that transcutaneous spinal
direct current stimulation (tsDCS) has
neuromodulatory effects on spinal motor circuitry
(e.g. Bocci et al., 2014). There is currently only one
computational study of the electric field distribution
during tsDCS applied on the thoracic spine region that
has been published, which applies realistic human
models based on high-resolution MRI of healthy
volunteers (Parazzini et al., 2014). There are no known
tsDCS modelling studies on human cervical spine, so
there is a need to develop human realistic models to
solve for the field distribution in cervical spine
stimulation.
The main objective of the present study is to
perform a finite element analysis (FEA) of the electric
field distribution in tsDCS in the cervical spine region,
and refer to cervical spine circuitry that may be
modulated by tsDCS in order to address viability for
clinical application purposes.
2 METHODS
The 34 year-old Duke model, from the Virtual
Population Family v1.x models (Christ et al., 2010),
was used to generate volume meshes for FEA with
MIMICS (v16, http://www.materialise.com/mimics).
From this model, eight tissues were selected: skin, fat
(including subcutaneous adipose tissue), muscle, bone
and vertebrae, intervertebral disks, dura mater,
cerebrospinal fluid (CSF) and spinal cord.
The electrode configuration tested followed the
experimental setup considered in Bocci et al. (2015):
the target electrode was placed over the C6-T1 spinous
processes; the return electrode was placed over the
right deltoid muscle, in a site far from the target. The
electrodes were modelled as 5x7 cm
2
rectangular
sponges soaked in saline solution (σ =2 S/m (Miranda,
Mekonnen, Salvador and Ruffini, 2013)), with a
thickness of 3 mm thick, between electrode and skin
surfaces. Electric conductivity value of all tissues
were based on data found in literature for DC currents:
σ
skin
= 0.435 S/m; σ
fat
= 0.040 V/m; σ
muscle
= 0.355 S/m
(average between muscle transverse (0.043 S/m) and
muscle longitudinal conductivity (0.667 S/m) values
from Rush, Abildskov and McFee, 1963); σ
vertebrae/bone
= 0.006 S/m; σ
intervertebral disks
= 0.200 S/m; σ
dura mater
=
0.03 S/m; σ
CSF
= 1.79 S/m; σ
spinal cord
= 0.154 S/m
(Geddes and Baker, 1967; Rush, Abildskov and
McFee, 1963; Haueisen, Ramon, Eiselt, Brauer and
Nowak, 1997; Baumann, Wozny, Kelly and Meno,
1997; Struijk, Holsheimer, Barolat, He and Boom,
1993). COMSOL Multiphysics (version 4.3b,
www.comsol.com) was used to calculate the electric
field distribution using FEA. The current intensity in
the electrodes was set to 2.5 mA, as in previous studies
(Bocci et al., 2014). The boundary conditions were
applied according to Miranda et al. (2013).
3 RESULTS
Figure 1 presents the electric field magnitude
distribution along the spinal cord. Figure 1a) shows
the volume-weighted average along the z axis,
considering slices of the spinal cord with 1 mm
height. This curve has two peaks in the region of the
spinal segments C5-T1 (corresponding to the braquial
plexus), with values of 0.171 V/m (z = [1570;1571]
mm) and 0.186 V/m (z=[1587; 1588] mm). Figure 1b)
shows the electric field magnitude volume
distribution in the spinal cord, where it can be seen
that these and other local maxima in the field
distribution are found in regions where the vertebral
Fernandes, S., Salvador, R., Wenger, C., Carvalho, M. and Miranda, P..
Transcutaneous Spinal Direct Current Stimulation - Modelling the Electric Field Distribution in the Cervical Spinal Cord.
Copyright
c
2015 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
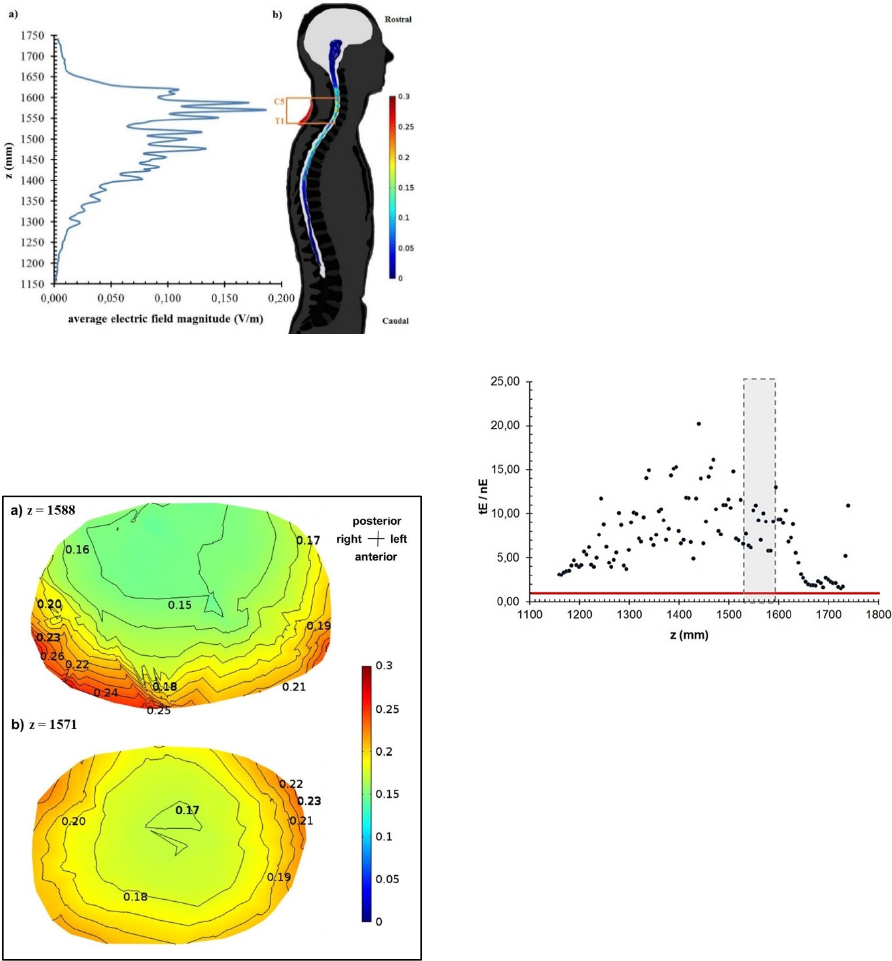
canal is narrowed due to the intrusion of
intervertebral disks or vertebrae body’s edges.
Figure 1: a) Volume-weighted average electric field
magnitude distribution along the z axis; b) Electric field
magnitude volume distribution colour plot in the spinal
cord, including a background representation of anatomical
structures, the target electrode (in red) and the position of
the brachial plexus spinal segments C5-T1 (in orange).
Figure 2: Contour plots of the electric field magnitude
(V/m) in the spinal cord in two axial slices: a) z=1588 mm;
b) z=1571 mm. The anatomic orientation and the colour
scale (in V/m) are presented on the right.
Figure 2 presents two contour plots of the electric
field distribution in the spinal cord at the level of the
two highest peaks in fig. 1a). In Figure 2a), the
maximum values reach 0.26 V/m and are located in
the anterolateral region of the spinal cord, with an
asymmetric distribution with a larger maximum
magnitude region on the right. This could be due to
the position of the return electrode, influencing the
electric field lines direction. Figure 2b) presents a
more symmetric distribution, with maximum field
magnitude values on the posterolateral region of the
spinal cord, reaching a maximum of 0.23 V/m. The
fact that Figure 1a) presents an average distribution
accounts for the difference in maximum value peaks
when comparing with Figure 2.
Values of the ratio between the magnitudes of the
normal and tangential components to the spinal cord
surface as function of position along the z axis are
presented in Figure 3. The values calculated span
between 1.5 and 20.2, which means that the tangential
component is higher than the normal component,
resulting in an electric field direction preferentially
tangential to the spinal cord.
Figure 3: Ratio between the magnitudes of the tangential
and normal components of the electric field along the z axis
in the spinal cord. The red horizontal line corresponds to a
ratio of 1 (equal magnitude of both components). The
region of the segments C5-T1 is marked in the plot area in
grey.
4 DISCUSSION
In modelling studies of tDCS, based on the
stimulation conditions applied to the motor cortex in
clinical studies with neuromodulatory effects
reported, electric field magnitudes of 0.15 V/m and
above were registered (Miranda et al. 2013). In the
present study, the electric field magnitude distribution
presented values above 0.15 V/m in the upper region
of the spinal segments C5-T1. As this region is related
to upper limb function (braquial plexus), this may
indicate that the values reached can be sufficient for
neuromodulatory effects on upper limb neurological
functions. Figure 2a) presents maximum values in the
anterolateral region of the spinal cord. This region is
related with sensory ascending tracts, responsible for
proprioception (spinocerebellar tracts), traditional
senses (spinothatamic tracts), and motor
subconscious descending tracts that regulate balance,
muscle tone, eye, hand and upper limb position. In
Figure 2b), the maximum values are located mainly
on the posterolateral region, related to tracts that
regulate conscious (posterior and lateral costicospinal
tracts) and subconscious (rubrospinal tracts) control
of skeletal muscles. These results are in agreement
with exploratory clinical tsDCS studies, that show
modulation of nociceptive ascending pathways and
spinal motor circuitry, depending on electrode
polarity, when stimulating thoracic and cervical spine
regions (Cogiamanian et al., 2012; Hubli, Dietz,
Schrafl-Altermatt and Bollinger, 2013; Bocci et al.,
2014). In particular, cervical cathodal tsDCS had an
increasing effect in motor unit recruitment and
decreased peripheral silent period in respect to sham
and anodal conditions (Bocci et al., 2014).
In the present study, the electric field in the spinal
cord had a larger tangential electric field component
along the spinal cord. As the electric field is directly
proportional to the current density, this may be in
agreement to the results of the modelling study by
Parazzini et al. (2014), in which the current density
direction in the spinal cord was mostly longitudinal
during thoracic tsDCS.
One shortcoming of the present model is the low
number of tissues, considering only the ones closer to
the target electrode. A more complete model could
reveal more about spreading effects on the electric
field. Also, the muscle conductivity value was taken
as an average between transverse and longitudinal
values in the literature, so anisotropic data could be
valuable in future studies. In spite of these limitations,
the results are in agreement with previous modelling
and experimental results.
Cervical tsDCS is a promising non-invasive
clinical tool for neuronal circuitry modulation in the
cervical spinal cord. It could address neuronal
dysfunctions like spasticity, present in many
neurologic diseases (e.g. amyotrophic lateral
sclerosis). Defining accurate models that predict the
physical effects of tsDCS on spinal neurons could be
a powerful tool to develop clinical applications more
focused on the specific neurologic patient needs.
ACKNOWLEDGEMENTS
This research was supported in part by the
Foundation for Science and Technology (FCT),
Portugal. S. R. Fernandes was supported by a FCT
grant, reference SFRH/BD/100254/2014. C. Wenger
was supported by Novocure.
REFERENCES
Baumann, S. B., Wozny, D., Kelly, S., Meno, F. (1997).
The electrical conductivity of human cerebrospinal
fluid at body temperature.” IEEE Transactions on
Biomedical Engineering, 44(3):220-223.
Bocci, T., Vanninia, B., Torzini, A., et al. (2014). Cathodal
transcutaneous spinal direct current stimulation
(tsDCS) improves motor unit recruitment in healthy
subjects. Neuroscience Letters, 578:75–79.
Christ, A., Kainz, W., Hahn, E. G., et al. (2010). The Virtual
Family-development of surface based anatomical
models of two adults and two children for dosimetric
simulations. Phys. Med. Biol., 55:N23–N38.
Cogiamanian, F., Ardolino, G., Vergari, M., et al. (2012).
Transcutaneous spinal direct current stimulation.
Frontiers in Psychiatry, 3, article 63.
Geddes, L.A. and Baker, L.E. (1967). The specific
resistance of biological materials – a compendium of
data for the biomedical engineer and physiologist. Med.
& Biol. Eng., 5:271 – 293.
Haueisen, J., Ramon, C., Eiselt, M., Brauer, H., Nowak, H.
(1997). Influence of tissue resistivities on
neuromagnetic fields and electric potentials studied
with a finite element model of the head. IEEE Trans.
Biomed. Eng., 44:727-735.
Hubli, M., Dietz, V., Schrafl-Altermatt, M., Bollinger, M.
(2013). Modulation of spinal neuronal excitability by
spinal direct currents and locomotion after spinal cord
injury. Clinical Neurophysiology, 124: 1187-1195.
Miranda, P.C., Mekonnen, A., Salvador, R., Ruffini, G.
(2013). The electric field in the cortex during
transcranial current stimulation. Neuroimage, 70:48-
58.
Parazzini, M., Fiocchi, S., Liorni, I., et al. (2014).
Modelling the current density generated by
transcutaneous spinal direct current stimulation
(tsDCS). Clinical Neurophysiology, 125(11):2260-70.
Rush, S., Abildskov, J.A., McFee, R. (1963). Resistivity of
body tissues at low frequencies. Circulation Research,
12:40-50.
Struijk, J. J., Holsheimer, J., Barolat, G., He, J., Boom
H.B.K. (1993). Paresthesia thresholds in spinal cord
stimulation: a comparison of theoretical results with
clinical data. IEEE Trans. Rehab. Eng.,1:101–108.
