
Patient with Complex Needs - Experience in Implementation of
LSV-Carewell Platform
Kazimierz Frączkowski
1
, Antoni
.
Zwiefka
2
, Marcin Zaremba
2
and Krzysztof Sikora
3
1
Wroclaw Univesity of Technology, Wyb. Wyspianskiego 27, Wrocław, Poland
2
A. Falkiewicz Specialist Hospital, Warszawska 2, Wrocław, Poland
3
IMMD Health, Institute Medical Mobile Device, Krawiecka 3/10 Wrocław, Poland
Kazimierz.fraczkowski@pwr.wroc.pl, antoni.zwiefka@dolnyslask.pl, mar_cinzaremba@o2.pl,
krzysztof.sikora@immdhealth.com
Keywords: Home telemonitoring, mobile technology, chronic illnesses, quality of life
Abstract: The paper describes telecare procedure concerning patients aged between 65-85 years with at least 2 chronic
diseases including hypertension (ICD I10), diabetes (ICD E 11), chronic obstructive pulmonary disease (ICD
J44) or heart failure (ICD J50). Ultimately, the project will involve 100 patients qualified on the basis of
medical history (last stay in hospital) divided in two groups. In the qualified group, each patient must have
at least 60 points according to the Barthl scale. The first group comprises 50 patients taken care of by tele
monitoring, which was provided to them with measuring equipment to be able to assess the selected
parameters at home (depending on the chronic disease). Then, the results are transmitted automatically via
mobile phone network to LSV Telecare (Lower Silesia Voivodeship Telecare) system. The other group of
patients consists of those, who were not included in the home monitoring. They are provided with medical
care within the current Polish health care system. In this paper we present scenarios and models of business
processes, necessary to achieve the objectives of the Care Well project, which is implemented under
Competitiveness and Innovation Framework Programme 2007-2013 (project "Multi-Level Integration for
Patients with Complex Needs"; grant agreement no: 620983) . The project involves 13 partners from 8 EU
countries - project duration is 36 months. The technical parts of the project include tests and examinations of
the economic and social effects, as well as indicators of the quality of life based on ICT platform for
communication and exchange of medical data, that are essential in the treatment of patients qualified for
Telecare.
1 INTRODUCTION
In Poland, among the population of people over 65 a
man lives in good health, on average, to 74, and a
woman to 78. All scenarios predict that by 2050
percentage of the population aged 65 years and more
will double, ie. from 15.8% in 2013 to 31.3% in the
low scenario, and to 35.7% in the very high scenario.
In the same period the number of the aged 85 and
over, is expected to increase five times [http://
Stat.gov.pl/ obszary, 2015; Population Projection
2014-20150, Warszawa 2014].
In the last 50 years the number of people aged 60
and over has tripled and it is expected that it will
arrive triple again to 2 billion by 2050. In China, there
is a region, that the increase of the proportion of
people over 65 years is estimated to reach 22,7% by
2050 [Guy Pare, Mirrou, 2010]. Despite the
prolongation of life expectancy, people are not
healthy for longer time, on the contrary external
conditions and changes of the traditional model of life
are often the cause of the emergence of new and
chronic diseases of civilization (diabetes, heart
disease, hypertension etc.), a list of which according
to the Health Minister regulation from 2009 (Dz.Uz
2009, No. 212, item. 1647) in Poland consists of 41
items. And it is estimated that these diseases cause
about 60% of all deaths in almost all countries
[http://Stat.gov.pl/obszary, 2015; Population
Projection 2014-20150, Warszawa 2014].
122
FrÄ
ˇ
Eczkowski K., Zwiefka A., Zaremba M. and Sikora K.
Patient with Complex Needs - Experience in Implementation of LSV-Carewell Platform.
DOI: 10.5220/0005890501220128
In Proceedings of the Fourth International Conference on Telecommunications and Remote Sensing (ICTRS 2015), pages 122-128
ISBN: 978-989-758-152-6
Copyright
c
2015 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
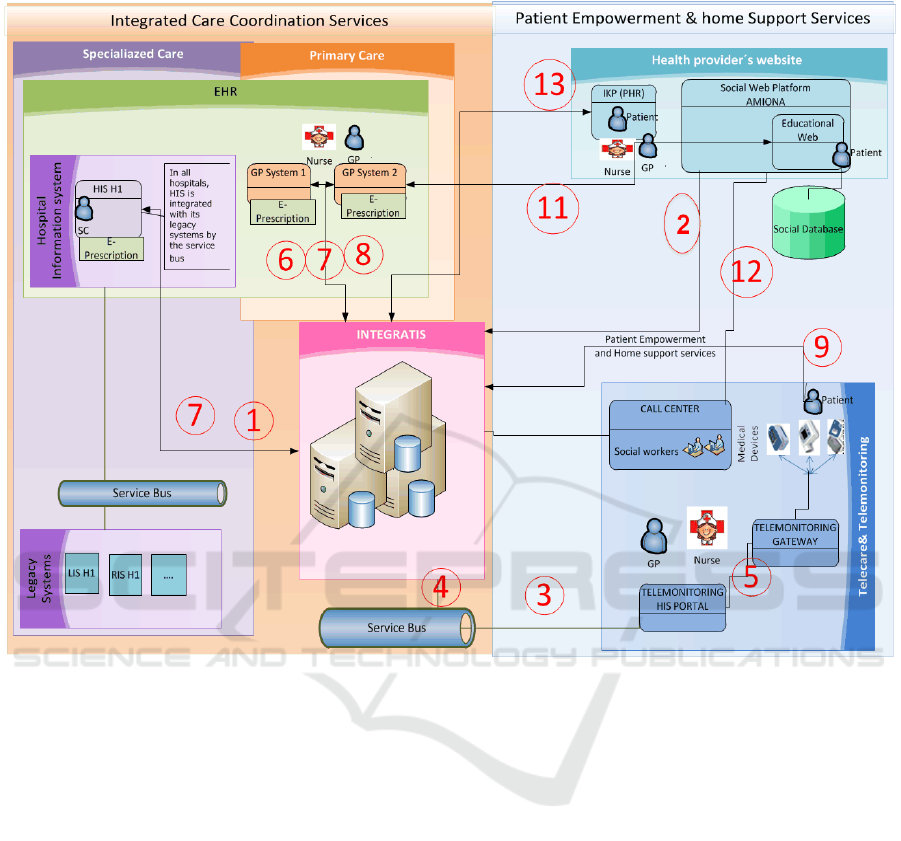
Figure 1: CareWell ICT Architecture Lower Silesia
That is why, there has been set up a number of
programs supporting frail patients aged 65+ with
more than one chronic disease. One of them is
CareWell project, which is aimed at improving the
efficiency and quality of medical care. The project is
carried out in a consortium with the participation of
13 partners from 8 EU countries (Spain, the United
Kingdom, Italy, Croatia, Germany, Belgium,
Poland). The main objective of the CareWell-LSV
project activities is to prepare ICT platform services
based on the modelled business processes, which
include 100 patients who are qualified on the basis of
the analysis of a disease history (discharge extract
from hospital).Therefore in many countries and
regions in Europe there are being implemented new
systems supporting telecare [Alan Wailer, Tony Maitby
2012]. A new innovative platform, created in Lower
Silesia Voivodeship (LSV), for telecare is based on
the integration of the already developed applications.
In LSV TELECARE the results from mobile
measuring devices are collected in Monitoring
Platform and automatically transmitted to the
Integration Platform
To ensure a better quality of life for people aged
65+ with chronic diseases, it is needed to bear the
economic burden of chronic diseases, which
represents 46% of the global burden caused by these
diseases. This phenomenon may be supported by
information, communication technologies - mainly
mobile ones, and that is an area called m-Health.
All possible questionnaires (including Bartel
scale) have been in CareWell Platform within
Integration Platform.
A qualified group of patients must have not fewer
than 60 points by Barthel Scale. The first group of 50
patients that will be tele monitored, has been provided
with measuring device for measuring the selected
parameters at patients home. After the measurement
is completed the results, are sent (via mobile
Patient with Complex Needs - Experience in Implementation of LSV-Carewell Platform
123

telephone network) to the CareWell Lower Silesia
System. The other group of 50 patients is not
monitored at home and they are provided with
medical care within the system according to the
current health care model in Poland.
2 SYSTEM ARCHITECTURE
AND FUNCTIONAL
EXPERIENCE
Key aspects in the design of a modern system of
telecare is the integration of technological solutions,
and the existing information systems, as well as the
applicable procedures of patient care with mobile
technologies in telecare. These issues are the subject
of numerous works on computer systems and their
clinical effects [Guy Pare and all, 2010, Guy Pare and
all 2010, Spyros Kitsiou and all 2015]. These actions
should find a way to show the benefits and how to
teach the end user, that is a patient, how to operate
mobile measuring devices at home. Therefore, the
platform building design involves the integration of
three sub platforms:
Educational - Information Platform (Social)
Integration Platform (Service Buss)
Monitoring Platform
As Lower Silesia currently does not have many IT
systems implemented to support the delivery of care
or share information, both CareWell pathways will be
significantly improved with the proposed ICT-
enabled services and functionality.
The development of a platform, presented in
Figure 1, is to provide interoperability between
different IT systems used in primary and secondary
care. It will enable information to be shared by
various care practitioners and patients within new
functionalities:
1. Registration of patient referrals for home care
and telemedicine. This is the first task in the
LSV Telecare platform.
2. Logged user access to the Information -
Education Portal and to Integration Platform.
3. Patients Registry Update Service in HIS by
Integration Platform.
4. Service of research results transfer by HIS
Patient Portal to Integration Platform.
5. Registration of the performed patient results
in HIS Portal.
6. GPs access to EHR and their tasks supporting
LSV Telecare procedure.
7. Nurses access to EHR, and their tasks
supporting LSV Telecare procedure.
8. Patients access to their own EHR and their
tasks supporting the process of LSV Telecare
procedure.
9. Implementation of developed services at the
country level, like e-Prescription (P1 Project)
within LSV Telecare procedure.
10. Call Centre staff access to their own tasks
supporting LSV Telecare procedure. -
receiving e-mail and SMS alerts.
11. Doctor, nurse and patient access to the
Education – Information Portal.
12. Call Centre staff access to the and Education-
Information Portal.
13. Some of the developments and changes
which will revolve around the new
interoperability of Integra TIS system.
Each of the above mentioned systems functionally
meets the requirements of the identified key aspects.
The Monitoring Platform is responsible for operation
of measurement devices. The most important issue is
the reliability of measurements in the context of user
authentication. It is unacceptable to assign mistaken
measurements to a patient. On the one hand, suitable
authentication, authentication and data security, and
on the other hand, greater ease of use and reliability.
To meet these requirements it is necessary to take
these constraints into account at the stage of
designing a subsystem, which is supposed to manage
its tasks.
The next subsystem is an Integration Platform
whose main task is to integrate all the subsystems and
enable their use to cover specific requirements which
are put before the health care system in the region
(country). At the stage of preparation for
implementation there are identified requirements,
which are then transferred into the BPM process
model.
This model is consulted with specialists and then
their approval is followed by the implementation of
telecare process. The Integrated Platforms mainly
task is to take care of the implementation of telecare
in accordance with the modelled procedures and
allow for an adequate response in any situation.
Another task of the Integration Platform is the storing,
processing and sharing of EHR.
Fourth International Conference on Telecommunications and Remote Sensing
124
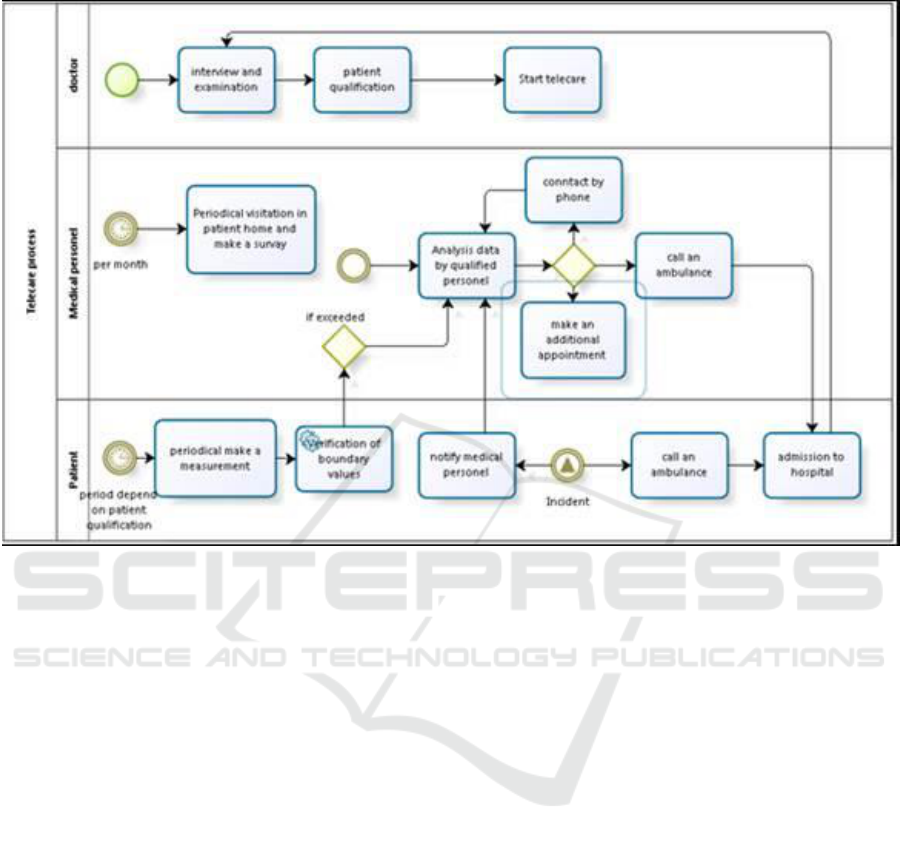
Figure 2: CareWell Homecare process model
Another aspect, which is equally significant as the
previous ones, is to enable patients to benefit from
telecare services in a safe way that they can
understand. Facing the problem of an aging
population and the fight against social exclusion, it
becomes increasingly important to educate the public,
create the opportunity for people to learn about and
understand a model of telecare and the benefits it
brings. The most important task, as well as most
difficult one to be completed by that sub platform is
to educate patients make them aware that the use of
telecare increases their safety and a quality of life.
Confronted with the standard model of health care,
telecare give you more benefits. Social portal
functionality also means to patients an easy access to
their care history (of the disease), the possibility of
being kept informed with their results and the feeling
of having more control over the process of health
care.
2.1. BUSINESS PROCESS MODEL
FOR LSV - TELECARE
The first step in implementation of LSV - TELECARE
is suitable qualification of patients and, then
depending on its outcome configuration of the
appropriate telecare procedure. This is important
because the process of telecare which is implemented
in the system, described crucial flow of information
and tasks, but does not define how various steps have
to be performed by individual patient
The telecare process of the Lower Silesia
CareWell System assumes that at fixed intervals a
patient will perform life parameters measurements at
home and the results will be transferred to a
healthcare unit. In contrast to the old style home care,
the telecare results have to be checked by a doctor
who has to determine what specific tests and at what
intervals the patient should pursue. Of course, during
the process there may be a need to change some
details such as measurements distance.
While the results of the patients’ measurements
flow into the central system, algorithms analyze the
results and examine whether they exceed the
thresholds, and check if their behaviour is similar to
the expected one. If there is a record of a departure
from the norm, in the system appears a task of
analyzing these results by hospital staff - in our case,
by a nurse.
Her task is to verify whether the test was carried
out in a correct way, whether the patient at that time
Patient with Complex Needs - Experience in Implementation of LSV-Carewell Platform
125

may have taken any medicine responsible for the
distortion of the results or if his behaviour did not
affect their values (e.g. increased physical activity).
When the observed anomaly is an erroneous
measurement or it is caused by a human error, the
patient is recommended to repeat the test. If it is a
worrying signal which may endangers the patient's
health a nurse can contact a doctor or intervene
immediately by calling an ambulance to the patient.
And here we meet another phenomenon described
in telecare procedure that is intervention, which we
understand as the situation, that is caused by an
undesired phenomenon (e.g. accident) or it is a
significant deviation from the standard
implementation of the procedure. The incident may
be reported by the patient in two ways. In the first the
patient using the supplied phone numbers call the Call
Center (in hospital conducting this procedure), where
he can obtain help from a nurse. In some situations, a
nurse may consult it with the doctor. She can also
arrange a home visit earlier, or in special situations
call an ambulance to the patient. The situation in
which a patient calls the emergency room directly is
considered to be an incident. Then he is admitted to
hospital according to standard procedures that he
undergoes, and after their completion (after begin
discharge) the patient record is supplemented with an
extract from hospital.
In the course of the procedure there are also
anticipated periodic visits by a nurse in the patient
home. Normally this is done once a month. Although
in case of incidents appearance, their frequency can
be increased.
Once the telecare goal is reached, a patient visits
a doctor, who may decide to continue the treatment or
end the procedure. In the case of telecare procedure
termination, there is generated an automatically
record of results and doctor prepares a detailed report
for the whole period covered by telecare.
2.2 Stable Patients – out of hospital
care
The implementation of the LSV teleCare integrated
pathway will enable the following developments to
the service model:
Better understanding of the roles and
responsibilities of the different care practitioners
involved in delivering services and interventions
within the care pathway.
Integrating the hospitalisation of those patients
who require it as part of the care pathway to provide
better patient care transition experiences across the
different sectors and professionals.
Introduction of telemonitoring for patients who
require this service.
Easier access to healthcare response service for
patients through the platform.
Electronic Case Record (ECR) will provide an
improved communication mechanism through on
email box, and thus enhance the co-ordination of a
patient’s care.
The platform will provide a directory of services
for patients, family members and informal care
givers, as well as professionals, to search for
appropriate quality of the assured health and
wellbeing services that are available.
Patients will be able to access the e-Prescription
and choose their dispensing pharmacy.
2.2.1 Unstable Patients – out of hospital care
The above enhancement for the ‘stable’ patient will
also be relevant for the ‘unstable’ patient. In addition,
virtual consultations will be able to be activated, if
necessary, among hospital specialists, nurses and GPs
via email box when a patient’s health and wellbeing
deteriorates.
2.2.2 Inpatient - hospital care
The hospital information system (HIS) should be
integrated by the ECR; healthcare professionals will
have access to the information (anonymised) in the
Platform if a patient gets admitted. Selected doctors
involved in CareWell will have access not only to the
information in HIS, but also to LSV CareWell
Platform. If a doctor is interested in the information
uploaded by the patient, they will ask permission
from the patient to look at this data. This should
provide improved information on the patient’s
medical history and the events leading up to hospital
admission.
The educational platform in this phase of the
project is not targeted at hospital doctors, but they
will be able to access the information in the platform
if they are interested in it.
2.2.3 Inpatient – hospital discharge
preparation
The hospital will be able to refer the patient for
telemonitoring if they are not already receiving the
intervention according to the defined CareWell
criteria, and determine their physiological parameters
and frequency accordingly. In addition, patients will
be signposted to appropriate patient empowerment
services and educational content through the
platform.
Fourth International Conference on Telecommunications and Remote Sensing
126

For patients who were receiving telemonitoring
prior to their admission, it is expected that they will
return to receive the telemonitoring service upon
being discharge from hospital.
3 MATERIAL AND METHOD
The main Project action was preparation of the
material for the Ethics Committee, which was
supposed to agree on a research project. At the
meeting of the Ethics Committee, in accordance with
the applicable legislation, it was requested to prepare
a proposal to the Bioethical Committee containing:
1. CV of the principal researcher
2. A detailed description of the project
3. A written acceptance of managers of the centers
where the examinations are performed
4. Consent of a trustee of archival material to its use
(each center)
5. An assessment card of assessment of a service
beneficiary directed to a care / staying in a care
unit (rating performed by service provider
according to the Barthl scale)
6. The information model for the participants of the
examination-doctor
7. The information model for the participants of the
examination-patient
8. Specimen of the informed consent of participants
or legal representatives to participation in the
examination and data processing related to this
participation (in the study).
9. Statement of the applicant about the knowledge
of principles of medical confidentiality
10. Submit a policy of obligatory liability insurance
of the entity engaged in medical activities.
Another element of the work in the project design
was based on modelled telecare processes presented
in Figure 2 and then selection of the suppliers of
technical solutions, ie. Platform, which is shown in
Figure 1. The integration and implementation work
followed by training of medical staff - doctors and
nurses, lasted until 15.06.2015r.
Individual technical means for patients include a set
of 50 mobile phones, smartphones L65 LG (LG-
D280n) for each patient. And in addition:
A Diabetic set - glucometer- ProfiLine Blutzucker-
Messsystem - 20 pcs.
B. COPD set - pulse oximeter PC-60NW - 5 pcs.
and peakflow meter - Asma-1 Vitalograph company
C. Hipertension set - Blood pressure meter -
SeniorLine BT model TD-3128- 15 pcs.
D. Set heart failure – pulse oximeter PC-60NW,
weight scales - 10 pcs.
Patients of Group A (diabetes) receive the
glucometer.
Patients of Group B (POHP) will receive
peakflowmeter and pulse oximeter.
Patients of Group C (hypertension) will
receive blood pressure meter.
Patients of Group D (heart failure) receive
pulse oximeter and a weight scales.
Ultimately, the care will be provided for 100
people. The criteria for inclusion of patients into the
follow-up observation are age 65-85; combination of
not fewer than 2 types of diseases: hypertension (ICD
I10), diabetes (ICD E11), chronic obstructive
pulmonary disease (ICD J44), heart failure (ICD I50).
Another required condition is to obtain at least 60
points according to Barthl scale.
Among the exclusion criteria involved in the
project there were established: age below 65 and
above 85 years, obtaining fewer than 60 points on a
Barthl scale, previous myocardial infection or stroke,
ischemic or haemorrhagic, in the last three months, an
active process of cancer of any location, mental
illnesses and unintentional loss of body weight: BMI
<19, or weight loss ascertained by a doctor.
Qualifying took place on the basis of analysis of
the information card of hospital stay. Patients
qualified for observation were divided into two
groups. The target size of both groups is 50 people. In
Group I there were qualified persons covered by
telemonitoring, who were provided with measuring
devices depending on the disease entity. In Group II
there were enrolled patients who were not covered by
telemonitoring and who received no measuring
devices.
Both in Group I and Group II there are patients
with a similar disease profile, age group and degree
of disability. In the next stage, persons qualified for
the observation will be evaluated by a nurse
(assessment of vital signs, including efficiency by
Barthl scale) and a Primary Care physician during
scheduled visits in the clinic. The estimated time of
follow-up for individual patients is 18 months.
Until 06.30.2015 in the Project there were
enrolled 75 people, including 39 women representing
52% of the respondents, and 36 men respectively,
48% of respondents. The average age being 73.96
years. The most commonly diagnose disease entity is
hypertension (ICD I10) 73 persons (97.3% of the
respondents). 45 patients (60%) were diagnosed with
Patient with Complex Needs - Experience in Implementation of LSV-Carewell Platform
127

diabetes (ICD E11), and respectively in 15% (12
individuals) – with COPD chronic obstructive
pulmonary disease, patients and 9 patients (12%) -
chronic heart failure.
4 CONCLUSIONS
Key experiences and lessons that we gained at this
stage of the project include:
1. Difficulty in understanding and reaching
consensus on telecare model, which was then
mapped in the implemented user interface
2. Integration of telemedicine devices to be
available to patients with the platform (two
providers of technical components Germany and
Poland) to solve the problems of interpretation.
3. Overcoming the resistance in terms of a new type
of telecare service - bath on the side of the
organization (main beneficiary) and the method
of patient enrolment for the project.
4. Difficulties at the level of patient care in a
hospital - lack of willingness to understand the
scale of the problem, potential benefits for the
geriatric patient resulting from the "no standard"
way of medical care
5. Difficulties in convincing patients to the
unknown and so fare not processed of providing
medical assistances to geriatric patients in Polish
conditions
6. We observe and share the opinions of other
researchers that it is necessary to make further to
make the technology and service of measuring
devices in patients home easier, more intuitive
and requiring minimal action on the part of the
patient.
REFERENCES
Alan Wailer, Tony Maitby.: Actice again: A strategic policy
solution to demographic ageing in the European Union.
International Journal of Social Wekfare. Volume 21,
Issue Supplement s.1. pages s117-s130, October 2012.
http://stat.gov.pl/obszary-tematyczne/ludnosc/ludnosc/
sytuacja-demograficzna-osob-starszych-i-
konsekwencje-starzenia-sie-ludnosci-polski-w-
swietle-prognozy-na-lata-2014-2050,18,1.html
Population Projection 2014-2050. Central Statistical
Office, Warsaw 2014.
Guy Pare, Mirrou Jaana, Claude Scott., Systematic Revew
of Home Teleomitorin for Chronoc Diswases: The
Evidence Base. J Am Med. Inform Assoc. 2010 May-
Jun : 14 (3) : 269-277
Guy Pare, Khali Mogadem, Gilles Pineau, Carole St-
Hilare.: Clinical Effects of Home Telemonitoring in the
Context of Diabestes, Astma, Heart Failure and
Hypertensinon: A Systematic Review. J <ed
International Res. 2010. Apr-Jun; 12(2)
Spyros Kitsiou, Guy Pare, Mirou Jann.: Effect of Home
Telemonitoring Intervention on Patient witch Cronic
Heart Failure: An Overview of Systematic Reviews.,
Jurnal of Medical Internet Research, vol 17.no 3 (2015)
Fourth International Conference on Telecommunications and Remote Sensing
128
