
Low Cost 3D-Printed Biosensor Arrays for Protein-based Cancer
Diagnostics based on Electrochemiluminescence
James F. Rusling
1,2,3,4
, Karteek Kadimisetty
1
, Spundana Malla
1
,
Gregory W. Bishop
1
and Jennifer E. Satterwhite-Warden
1
1
Department of Chemistry, University of Connecticut, Storrs, Connecticut 06269-3060, U.S.A.
2
Institute of Materials Science, University of Connecticut, Storrs, Connecticut 06269-3136, U.S.A.
3
Department of Surgery and Neag Cancer Center, University of Connecticut Health Center,
Farmington, Connecticut 06030, U.S.A.
4
School of Chemistry, National University of Ireland at Galway, Galway, Ireland
Keywords: 3D-Printing, Cancer, Multiplexed Protein Detection, Microfluidics, Immunoarray.
Abstract: Development and fabrication of bioanalytical devices by 3D printing offers revolutionary new routes to low
cost clinical diagnostic devices for molecular measurements. Relevant to future protein-based cancer
diagnostics, we describe and review here our recent development of prototype protein immunoarray devices
using desktop Fused Deposition Modeling (FDM) and stereolithographic 3D printers. All these system
feature sensitive electro-optical detection by a method called electrochemiluminescence (ECL). Our first
3D-printed immunoarray features screen-printed sensors in which manual manipulations enable gravity flow
reagent delivery for measurement of 3 proteins at detection limits of 0.3 to 0.5 pg/mL. ECL detection is
achieved in an open channel on integrated disposable screen-printed sensor elements. We then address the
issue of printing and processing optically clear plastic using a stereolithographic printer to build a closed
ECL detection chamber. Finally, we describe a prototype 3D-printed microprocessor-controlled enclosed
microfluidic ECL immunoarray featuring reagent reservoirs, micropumps and clear plastic detection
chamber with printed nanowells for ECL emission.
1 INTRODUCTION
Desktop 3D printers offer unprecedented new
options to design and fabricate low cost, high
performance biosensors (Gross, B.C., et al., 2014).
Development of microfluidic sensing devices by 3D-
printing can provide rapid computer-based design
prototyping and testing, avoiding the necessity for
masks or templates used in more traditional
approaches such as lithography. Design-to-device
fabrication can be rapidly achieved with 3D-printers,
and devices can be produced cheaply without the
need for economies of scale. Recent examples
include 3D printed systems for monitoring metal
ions (Su et al., 2014) and add-ons for turning
smartphones into food allergen sensors (Coskun &
Wang, et al., 2013; Wei , Nagi, et al., 2014; Coskun
& Nagi, et. al, 2013; Roda et al., 2014; Wei, Luo, et
al., 2014). Electrochemical sensing was integrated
into 3D-printed fluidic devices for dopamine, nitric
oxide (Erkal et al., 2014) and hydrogen peroxide
(Bishop et al., 2015). Biological and diagnostic
applications have recently been reviewed (O’Neill,
et al. 2014; Meng, et al. 2015).
There is a high level of interest in the medical
community for measuring levels of multiple
biomarker proteins for cancer diagnostics (Hanash,
et al., 2011). Measuring biomarker proteins in
conjunction with genomic analysis of patients and
their cancers are expected to help usher in a new era
of Precision Medicine (Kohane, 2015). Serum levels
of proteins are biomarkers that can serve to indicate
the onset, existence or progression of cancer
(Hanash, et al., 2011, Rusling, et. al., 2011).
Measurement of panels of protein biomarkers holds
enormous potential for early cancer detection as well
as personalized cancer therapy and treatment
monitoring.
.
However, these applications have yet to
be broadly realized in a form that can be readily
adapted to point-of-care. For such diagnostic
strategies to reach widespread clinical or point-of-
care (POC) use, low cost, sensitive, easy to use
devices are needed to measure multiple biomarker
proteins in patient serum (Rusling, et al. 2010).
Rusling, J., Kadimisetty, K., Malla, S., Bishop, G. and Satterwhite-Warden, J.
Low Cost 3D-Printed Biosensor Arrays for Protein-based Cancer Diagnostics based on Electrochemiluminescence.
DOI: 10.5220/0005649000170022
In Proceedings of the 9th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2016) - Volume 1: BIODEVICES, pages 17-22
ISBN: 978-989-758-170-0
Copyright
c
2016 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
17

Enzyme-linked immunosorbent assay (ELISA) is the
gold standard for clinical protein assays with DLs as
low as 1-3 pg/mL, but with limitations in sensitivity,
analysis time, multiplexing, and sample size. Newer
commercial multiprotein detection systems are very
useful for research, and rely on expensive,
technically demanding instrumentation difficult to
implement in the clinic (Hanash, et al., 2011,
Rusling, et. al., 2011). These approaches rarely
achieve detection below pg mL
-1
levels, while some
biomarker proteins have serum levels well below 1
pg mL
-1
.
In this paper, we describe new approaches to
develop 3D-printed multiplexed protein
immunoassay devices using a sensitive electro-
optical detection method called
electrochemiluminescence (ECL) (Forster, et al.,
2009). Detection employs an ECL-active dye that
can be loaded into nanoparticle labels, and
electrochemically active co-reactant, and applied
voltage to produce visible ECL light detected by a
charge-coupled device (CCD) camera. Below we
describe a prototype 3D-printed immunoassay
system with screen-printed sensors in which manual
manipulations are used to enable gravity flow
reagent delivery for the detection of 3 proteins. This
system uses ECL detection in an open channel
without a window in front of the sensor elements.
We then address the issue of printing and processing
optically clear plastic to build a closed chamber that
will emit ECL light. Finally, we present a prototype
3D-printed microprocessor-controlled microfluidic
ECL immunoarray featuring reagent reservoirs and
clear plastic detection chamber with printed
nanowells for ECL emission.
2 RESULTS
2.1 Gravity-flow Immunoarray
A prototype protein immunoarray was fabricated
using the desktop Fused Deposition Modeling
(FDM) 3D printer MakerBot Replicator 2X and
polylactic acid (PLA). This device (Figure 1)
features an open channel housing a screen-printed
electrode array insert powered by a supercapacitor
for ECL generation detected by a CCD camera
(Kadimisetty, et al, 2016). The main array unit has
three 170 µL reagent reservoirs with sealing caps
connected to a common downstream microfluidic
channel (Figure 1). Solutions in the reservoirs flow
into and fill the 160 µL detection channel under
hydrostatic pressure. Initially, the insert caps seal the
reservoirs. Flow of sample and reagents commences
by removing the cap to drain the prefilled reservoir
into the detection channel. To run the assay the
operator releases the reagents in sequence by
removing the inserts.
A larger wash reservoir works with a lever-
activated platform that holds the sensor array, wash
reservoirs and waste tank at the bottom (Figure 1B).
Wash reservoirs also employ custom fit inserts to
turn flow on and off. Changing the lever to wash
position tilts the sensor array 25º to wash unused
immunoreagents to waste.
The sensors in the array have antibodies
attached to them to capture the protein analytes from
the sample. Assays proceed by allowing sample to
fill the detection chamber for an incubation period in
which antibodies capture the analytes, then
sequential washing, adding 100 nm RuBPY-silica-
antibody detection nanoparticles, washing, and
incubating. At this point the RuBPY-silica-antibody
particles have bound onto the sensors sites that have
previously bound analyte proteins in a sandwich
immunoassay. Finally TPrA co-reactant is added to
fill the detection channel and 1.2 V is applied by the
supercapacitor for 30 s. ECL light is initiated from
RuBPY in the silica nanoparticles by
electrochemical oxidation with TPrA co-reactant,
and light is detected by a CCD camera. The
supercapacitor is recharged using a small solar panel
and a cell phone light.
This immunoarray was tested by detecting three
prostate cancer biomarker proteins in serum. The
proteins were prostate specific antigen (PSA),
prostate specific membrane antigen (PSMA) and
platelet factor-4 (PF-4), and assays were completed
in 35 min. Detection limits of 0.3-0.5 pg mL
-1
for the
3 proteins in undiluted calf serum were found, and
the dynamic range is consistent with the levels of
these proteins in blood of cancer patients and
cancer-free individuals. Assays of 6 prostate cancer
patient serum samples gave good correlation with
conventional single protein immunoassays
(Kadimisetty, et al., 2016). Results suggest
successful 3D-printing of major components of a
very low cost portable immunoarray device (€0.90
in materials) with replaceable single-use electrode
array (€0.20 in materials) for sensitive, accurate
detection of proteins in biological samples. Assays
cost ~€0.50 each in expendable reagents. Power is
supplied by a portable Cellergy, 2.1 V, 80 mF
supercapacitor (€10) with a Sparkfun, 0.45 W, 94
mA solar panel (€12) for recharging. The entire
immunoarray with power supply costs ~€25, not
including the CCD camera. A drawback for point-
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
18

Figure 1: 3D-printed main array and wash reservoir module. (A) Basic array showing three reagent reservoirs equipped with
inserts along with flow path for reagents to reach microfluidic channel. (B) Wash reservoir module (1B Left) 3D model
showing freely moving lever to change between wash and load position along with wash reservoirs aligned with main array,
(1B Right) assembled immunoarray setup with both main array and wash module.
of-care (POC) applications is the lack of automation.
Nevertheless, this prototype suggests that 3D-
printing will be amenable to more sophisticated
immunoarray devices that can be automated
(Kadimisetty, et al., 2015).
2.2 Transparent 3D-Printed Devices
for ECL Detection
FDM printers produce opaque finishes unsuitable for
a closed optical detection chamber. Thus, we
designed and printed a prototype ECL sensor device
using a Form1+ 3D printer (Formlabs) and clear
methacrylate-based resin (Bishop, et al., 2015).
Uncured resin was removed by forcing isopropanol
through the device channels and then submerging in
isopropanol for 10 s. The device was polished using
abrasive papers, rinsed with water and dried, then
spray-coated with clear acrylic (Krylon, Cleveland,
OH) to achieve high clarity. Flow devices were
designed with 800 µm diam. channels featuring an
oval opening and screw-in inlet and outlet lines to
introduce solutions (Figure 3).
We first ascertained that the electrochemical cell
in this device gave theoretical voltammetry for
standard redox couples that was not influenced by
location in the flow channel. We then did simple
experiments to demonstrate ECL detection on
working electrodes through the clear plastic cell
windows. Oxidation of TPA leads to the formation
of cation radicals (TPA
●+
) and free radicals (TPA
●
)
that react with soluble [Ru(bpy)
3
]
2+
(as well as the
RuBPY-silica in the earlier example) to generate
electronically excited [Ru(bpy)
3
]
2+
* that emits ECL
light at 610 nm. The 3D-printed channel with
integrated electrodes was placed under a CCD
camera housed in a lightproof box to measure ECL
(Figure 4). At potential +0.95 V vs. Ag/AgCl,
images for 10 min exposure time were clearly
visible. Increasing concentrations of the
[Ru(bpy)
3
]
2+
in the reaction mixture gave increased
ECL. This simple device and experiments
established the technology to design and 3D print
ECL based biosensor arrays.
2.3 Prototype Automated 3D-Printed
ECL Immunoarray
We then developed a 3D printed array with
automated microprocessor controlled sample and
reagent delivery. Using the Form 1+ 3D printer we
printed a unibody optically clear ECL microfluidic
array (Figure 5A) with 5 reagent reservoirs leading
into a common microfluidic serpentine channel. The
channel addresses an underlying 32-microwell array
filled with upright single-wall carbon nanotubes
with attached antibodies for simultaneous detection
of multiple proteins (Figure 5B). The device is 6.5 x
3.0 x 0.5 mm (L x W x H) and takes 1.5 hours to
print at €1.2 per array. The maximum volume of
reagent chambers is ~150 µL and total volume of the
serpentine channel ~140 µL. Three micropumps are
connected to the 3 inlets of the array to pump sample
and reagents sequentially from the 5 chambers
(Figure 5A) to the detection channel to complete a
sandwich immunoassay. Complete automation is
achieved by programing micropumps with an
Arduino microcontroller to run the assay protocol.
The serpentine channel is 3D printed to be open on
one side with dimensions 1.2 x 0.15 cm L x W, and
350 µm thick. A tiny groove inside the channel
houses a stainless steel wire to serve as a counter
Low Cost 3D-Printed Biosensor Arrays for Protein-based Cancer Diagnostics based on Electrochemiluminescence
19
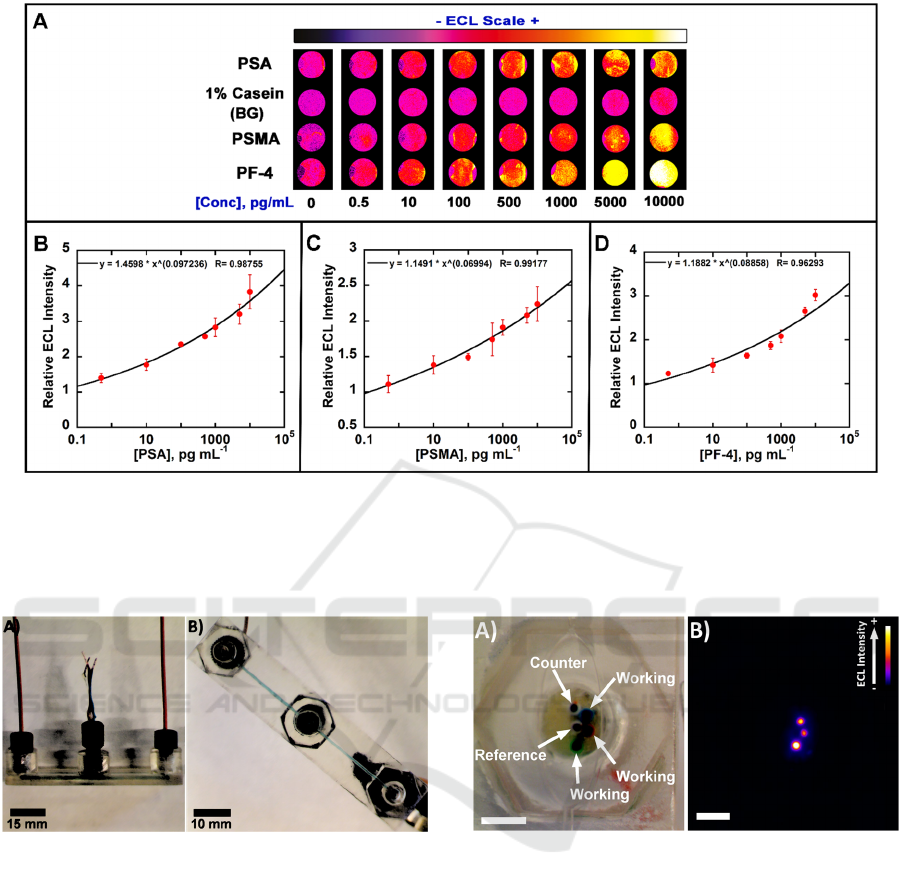
Figure 2: Calibration data from the 3D printed gravity fed immunoarray for 3 proteins in undiluted calf serum showing
influence of biomarker protein concentration on ECL response: (A) Recolorized ECL images of 8 arrays with showing
increase in ECL intensity with increased concentration. ECL signals digitized for (B) PSA, (C) PSMA and (D) PF-4 in calf
serum. Error bars show standard deviation for n = 4. Reprinted with permission from Kadimisetty, K., et al., 2016,
Copyright Elsevier 2016.
Figure 3: Clear 3D-printed fluidic device with
incorporated electrodes for ECL detection. A) Side view
equipped with threaded nuts and tubing for inlet/outlet
access to the 730 µm fluidic channel and a threaded nut in
the center through which Ag/AgCl reference and graphite
working and counter electrodes are integrated. B) Bottom
view of device, with electrodes on right. Reprinted with
permission from Bishop, G.W. et al., 2016, Copyright
Amer. Chem. Soc. 2016.
electrode. A pyrolytic graphite wafer was patterned
with microwells using an inkjet printer (Figure 1B)
as the working electrode to produce ECL. This wafer
was attached to the open side of the serpentine
channel using high tact silicone spray adhesive. The
resulting chip defines 32 microwells with 4 spots per
turn of the serpentine channel.
Prior to attaching the processed PG chip to the
array, upright single wall carbon nanotube forests
Figure 4: Photographs of 3-working electrode array
incorporated into the 3D-printed channel in figure 3. A)
Bottom view of 0.5 mm Ag/AgCl reference, 0.5 mm
graphite counter and three graphite working electrodes; B)
ECL response from electrode array in 180 µM
[Ru(bpy)
3
]
2+
in 0.2 M phosphate buffer with 100 mM
TPrA. Scale bars represent 3 mm. Reprinted with
permission from Bishop, G.W. et al., 2016, Copyright
Amer. Chem. Soc. 2016.
were grown in each microwell, followed by
chemically linking capture antibodies (Ab
1
) to the
carboxylated nanotube ends (Kadimisetty, et al.,
2015). This Ab
1
coated surface is then exposed to
incoming proteins in serum pumped by micropump
1 from chamber 1 during the assay (Figure 5A).
Then pumping stops for a 15 min incubation
followed by pumping wash buffer from chamber 2.
Later micropump 2 is initiated by the program to
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
20
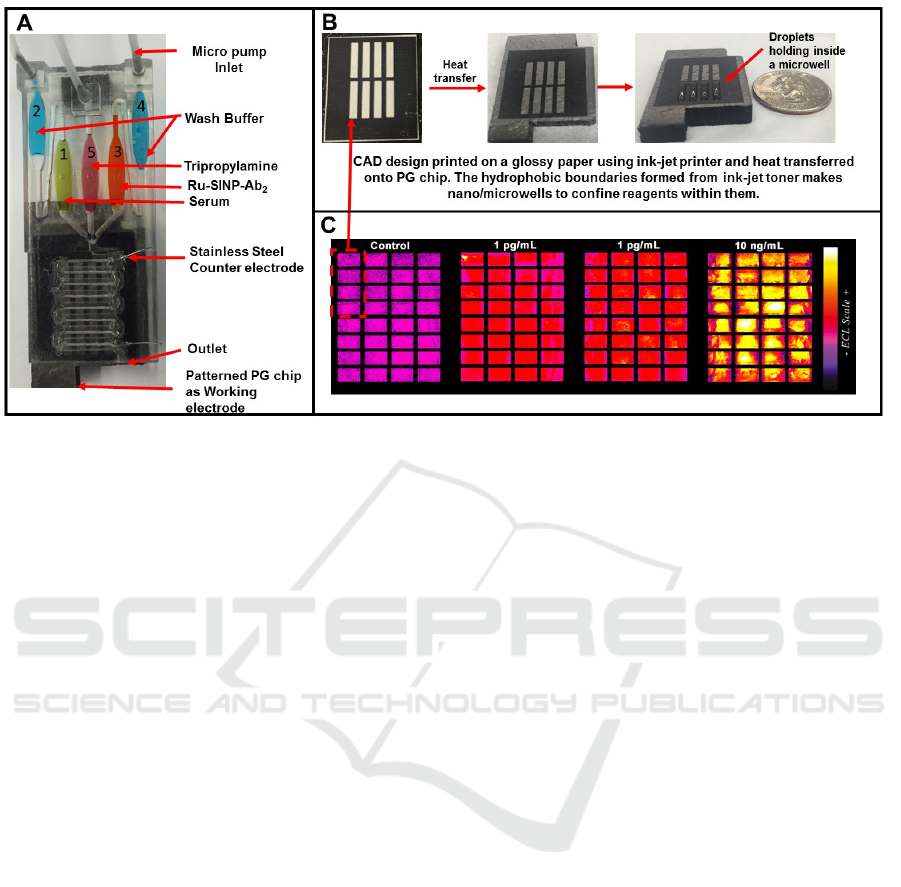
Figure 5: Prototype 3D printed automated immunoarray and proof-of-concept data: (A) Full array with reagent, sample and
buffer reservoirs, and serpentine channel covering a series of microwells for ECL generation; (B) the process of forming
microwells on the pyrolytic graphite wafer; (C) recolorized 4-array illustration of detection of prostate specific antigen in
serum at 0, 1 and 10 pg/mL PSA concentrations.
pump the 100 nm RuBPY-SiNP-Ab
2
detection beads
from chamber 3 to the array with captured proteins.
RuBPY-SiNP-Ab
2
are then incubated for 15 min,
followed by washing unused label particles using
wash buffer from chamber 4 to complete the Ab
1
-
protein-Ab
2
sandwich on the sensors. Then, the ECL
generating reagent (350 mM tripropylamine (TrPA)
with 0.05% Tween-20 (T20) and 0.05% Triton-X in
0.2 M phosphate buffer) is pumped into the
detection chamber from chamber 5. ECL is then
generated using a tiny Cellergy supercapacitor
applying 1.5 V for 120 s with light captured by a
CCD camera in a dark box.
Proof-of-concept experiments on this array
showed moderate reproducibility with RSD’s ≤ 17
% from spot-to-spot (n=32) and array-to-array ≤13
% (Figure 1C). Protocol and printing optimizations
are currently underway to improve these RSDs, and
to enable reliable multiplexing. Nevertheless, these
experiments establish that the automated 3D-printed
device can be used for relatively sensitive protein
detection. The entire immunoarray was built for
~€250 with three micropumps (€200), an Arduino
microcontroller (€30), a supercapacitor (€10) and a
3D printed array including the PG chip (€7). The
3D-printed component cost less that €1 and can be
disisable. The 3D-printed array can be disposable, or
regenerated and reused. The CCD camera is of
course reusable, but we are also exploring cheaper
alternatives.
3 CONCLUSIONS
Our exploratory work described above suggests that
low cost 3D printers provide excellent tools to build
the molecular diagnostic devices of the future. First,
we have fabricated a viable 3D-printed gravity fed
immunoarray to detect 3 proteins with better
detection limits that most commercially available
protein assays. Second, we have developed a
approach capable of fabricating closed microfluidic
devices that can measure ECL, and realized a
prototype automated 3D-printed immunoarray
capable of low concentration protein detection.
Future applications of the latter device are planned
for sensitive detection of 10 proteins in serum.
Universal protein-centered cancer diagnostics
promises to decrease overall cancer mortality by
earlier detection and molecular therapy monitoring
leading to better patient prognoses (Hanash, et al.,
2011, Rusling, et. al. 2010). However, widespread
translation of these technologies into the clinic will
require cheap, reliable, sensitive, automated
multiplexed protein detection devices. As we can
expect further advances in feature resolution and
speed (Tumbleston, J. R., 2015), 3D printing may
grow to become a major approach for fabrication of
bioanalytical measurement devices.
Low Cost 3D-Printed Biosensor Arrays for Protein-based Cancer Diagnostics based on Electrochemiluminescence
21

ACKNOWLEDGEMENTS
This work was supported financially by grants No.
EB016707 and EB014586 from the US National
Institute of Biomedical Imaging and Bioengineering
(NIBIB), NIH.
REFERENCES
Bishop, G. W., et al., 2015. 3D-Printed Fluidic Devices for
Nanoparticle Preparation and Flow-Injection
Amperometry Using Integrated Prussian Blue
Nanoparticle-Modified Electrodes, Anal. Chem. 87,
5437-5443.
Bishop, G. W., et al., 2016, Electrochemiluminescence at
Bare and DNA-Coated Graphite Electrodes in 3D-
Printed Fluidic Devices, ACS Sensors, in press.
Coskun, A. F., Wong, J., et al., 2013. A personalized food
allergen testing platform on a cellphone, Lab Chip. 13,
636-640.
Coskun, A. F., Nagi, R., et al. 2013. Albumin testing in
urine using a smart-phone, Lab Chip. 13, 4231-4238.
Erkal, J. L., et al. 2014. 3D printed microfluidic devices
with integrated versatile and reusable electrodes, Lab
Chip. 14, 2023-2032.
Forster, R. J., et al., 2009. Electrogenerated
chemiluminescence, Annu. Rev. Anal. Chem. 2, 359-
385.
Gross, B. C., et al., 2014. Evaluation of 3D printing and its
potential impact on biotechnology and the chemical
sciences, Anal. Chem. 86, 3240-3253.
Hanash, S. M., et al. 2011. Emerging molecular
biomarkers—blood-based strategies to detect and
monitor cancer, Nature Rev. Clin. Oncol. 8, 142–150.
Kadimisetty, K., et al., 2015. Automated Multiplexed ECL
Immunoarrays for Cancer Biomarker Proteins, Anal.
Chem. 87, 4472-4478.
Kadimisetty, K., et al. 2016, 3D-Printed Supercapacitor-
Powered Electrochemiluminescent Protein
Immunoarray, Biosens. Bioelectron., 77, 188–193.
Kohane, I. S. 2015. Ten Things We Have To Do To
Achieve Precision Medicine, Science. 349, 37-38.
Meng, C., et al., 2015. 3D printed microfluidics for
biological applications, Lab on Chip, 15, 3627-3637.
O'Neill, P. F., et al. 2014. Advances in three-dimensional
rapid prototyping of microfluidic devices for
biological applications. Biomicrofluidics 8, 052112.
Roda, A., et al., 2014. Integrating Biochemiluminescence
Detection on Smartphones: Mobile Chemistry
Platform for Point-of-Need Analysis, Anal. Chem. 86,
7299-7304.
Rusling, J. F., et al., 2010. Measurement of Biomarker
Proteins for Point-of-Care Early Detection and
Monitoring of Cancer, Analyst. 135, 2496-2511.
Su, C., Hsia, S., Sun, Y., 2014. Three-dimensional printed
sample load/inject valves enabling online monitoring
of extracellular calcium and zinc ions in living rat
brains, Anal. Chim. Acta. 838, 58-63.
Tumbleston, J. R., et al., 2015. Continuous liquid interface
production of 3D objects, Science, 347, 1349-1352.
Wei, Q., Nagi, R., et al., 2014. Detection and Spatial
Mapping of Mercury Contamination in Water Samples
Using a Smart-Phone, ACS Nano. 8, 1121-1129.
Wei, Q., Luo, W., et al., 2014. Imaging and Sizing of
Single DNA Molecules on a Mobile Phone, ACS
Nano. 8, 12725-12733.
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
22
