
Single Cell Array Impedance Analysis for Cell Detection
and Classification in a Microfluidic Device
Emre Altinagac
1
, Selen Taskin
2
and Huseyin Kizil
2
1
Department of Nanoscience & Nanotechnology, Istanbul Technical University, Istanbul, Turkey
2
Faculty of Chemical and Metallurgical Engineering, Istanbul Technical University, Istanbul, Turkey
Keywords: Microfluidics, Impedance Analysis, Lab-on-a-Chip, Single Cell Detection.
Abstract: Impedance analysis of single cells is presented in this paper. Following the separation of a target cell type
by dielectrophoresis in our previous work, this paper focuses on capturing the cells as a single array and
performing impedance analysis to point out the signature difference between each cell type. Lab-on-a-chip
devices having a titanium interdigitated electrode layer on a glass substrate and a PDMS microchannel are
fabricated to capture each cell in a single form and perform impedance analysis. MDA-MB-231 and HeLa
cells are used in our experiments.
1 INTRODUCTION
Microfluidic based cell separation and capturing
systems with high efficiency, fast response time,
multi-functionality, high accuracy and repeatability
rates are emerged as a new diagnosis tool for many
applications in biotechnology, drug discovery,
medicine, chemistry and environmental problems
(Mateo et al., 2014, Karabacak et al., 2014). They
require minimal sample size, low cost to fabricate,
portable and most importantly allow early detection
of circulating cancer cells and can be used as a
point-of-care product compare to macro-scale cell
separation and diagnosis systems (Alix-Panabieres et
al., 2014, Jin et al., 2014). In literature, active and
passive cell separation systems could be found on
the basis of differences in cell’s geometry, chemical
and electrical properties. Passive systems are based
on the flow characteristics of the fluid with particles
inside the microchannel and separation is
accomplished using the geometrical differences of
the particles, whereas active systems require outside
source, like magnetic field, electrical field, acoustics
and optics, to sort/capture the particle inside
microchannel. The outside source applied in the
active systems must accomplish the task without
damaging the viability of cells (Hajba et al., 2014).
Impedance spectroscopy (IS) is an important
measurement system in cell biology in the analysis
of cellular structure, cell physiology and cell to
disease interaction studies (Park et al., 2010).
Cellular resistivity measurement without the need
for any molecular marker can provide significant
information to researchers on the mechanisms of cell
functioning, especially in the formation and
progression of a disease. For example, membrane
specific capacitances of cancer tissue cells are
different than that of the normal cell membranes.
And it is well known that white blood cells have
different capacitance values among themselves, due
to the surface geometry of the cell membrane, and
cell membranes with specific apoptosis or necrosis
condition have different capacitance and
conductivity values compare to the ones at the
normal mode of operation. In a recent study (Anh-
Nguyen et al., 2016) long-term monitoring of MCF-
7 breast cancer cell attachment, adhesion, spreading
and the response of those cells to anticancer drug
Cisplatin is presented within the same platform.
These cellular activities and responses of cancer
cells to drug treatment are indicated by impedance
spectra of target cells. Another study (Dastider et al.,
2016) presents a biosensor for detection of low
concentration (39 CFU/mL) foodborne pathogen,
E.coli, on a microfluidic platform consist of two
dielectrophoretic focusing and impedance sensing
sequentially. Positive dielectrophoretic force applied
to concentrate the bacteria towards to the center of
the microchannel and anti-E.coli coated
interdigitated electrode arrays detects the flowing
bacteria throughout the microchannel.
Altinagac E., Taskin S. and Kizil H.
Single Cell Array Impedance Analysis for Cell Detection and Classification in a Microfluidic Device.
DOI: 10.5220/0006166700490053
In Proceedings of the 10th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2017), pages 49-53
ISBN: 978-989-758-216-5
Copyright
c
2017 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
49

The aim of our research is to fabricate a
microfluidic based lab-on-a-chip (LOC) device that
will consist of two main parts: In the first part, the
cells will be separated by dielectrophoresis (DEP)
based on their dielectric properties. In the second
part, cells are captured as single cell array by
hydrodynamic forces and the cell impedance is
measured. The fabricated LOC system has a
potential to be used for counting blood cells
(hemogram), stem cell count, drug resistance
detection, cell phenotype etc. These measurements
are currently made with restricted use of the flow
cytometer, which requires large volume of samples
which are fluorescently labelled and then excited by
the laser.
The two parts of our design are studied
separately to maintain the simplicity of our research
and this paper focuses on the capturing of target
cells by hydrodynamic forces and carrying out
impedance analysis for cell detection, classification
and characterization.
2 DESIGN
The idea is inspired from Tan’s study (Tan et al.,
2007) where target cells are trapped as a single cell
array throughout a two-dimensional microfluidic
channel. Interdigitated electrode couples are placed
under each trap site to detect captured cells via an
LCR meter. The width and the height of the
microchannel are close to the diameter of target cells
to maintain the stream of cells in a linear form.
There are two potential paths with different flow
resistances for cells to follow: Path 1 is the trap site
with a lower flow resistance when it is empty and
Path 2 is the fraction of the main channel. When a
cell is trapped in Path 1 consecutive cells are
directed to Path 2 due to increase in flow resistance.
Tan’s design consists of 5µm x 5µm narrow necks to
create a trap site in Path 1 and due to the fabrication
challenges of such structures we present a 3D
polydimethylsiloxane (PDMS) microchannel which
is shown in Figure 1 with a minimum feature size of
10µm. A narrow neck in Path 1 is created in vertical
direction by limiting the channel height to 5µm in
this region and the rest of the channel has a
thickness of 15µm.
Figure 1: There are two potential paths for cells to follow.
Path 1 has a lower flow resistance until a cell is trapped. A
triangular trap site has a lower flow resistance than a
circular one.
Different geometries for trap site are studied to
prevent multiple cell capturing in the same trap site
and clogging of main channel. The length and the
width of the Path 1 and Path 2 play a key role to
determine the flow resistance. Path 1 is designed to
be wider and shorter to maintain lowest flow
resistance possible. Fluid velocity variation is shown
in Figure 2 and it is shown that higher trapping
efficiency is achieved by just increasing the channel
length due to increase in the flow resistance of Path
2. But multiple cells could be captured at once in the
trap area in such structures.
Figure 2: Fluid velocity distributions for different Path 2
lengths. Colour bar: Fluid velocity (m/s).
We present a novel design to maintain capturing
single cells only in a graded triangular trap site as
seen in Figure 3. When a cell (1) is trapped in the
acute angled area the subsequent cells (2) are cleared
out of the trap site. This process repeats itself until
all the trap sites are filled with single cells.
BIODEVICES 2017 - 10th International Conference on Biomedical Electronics and Devices
50
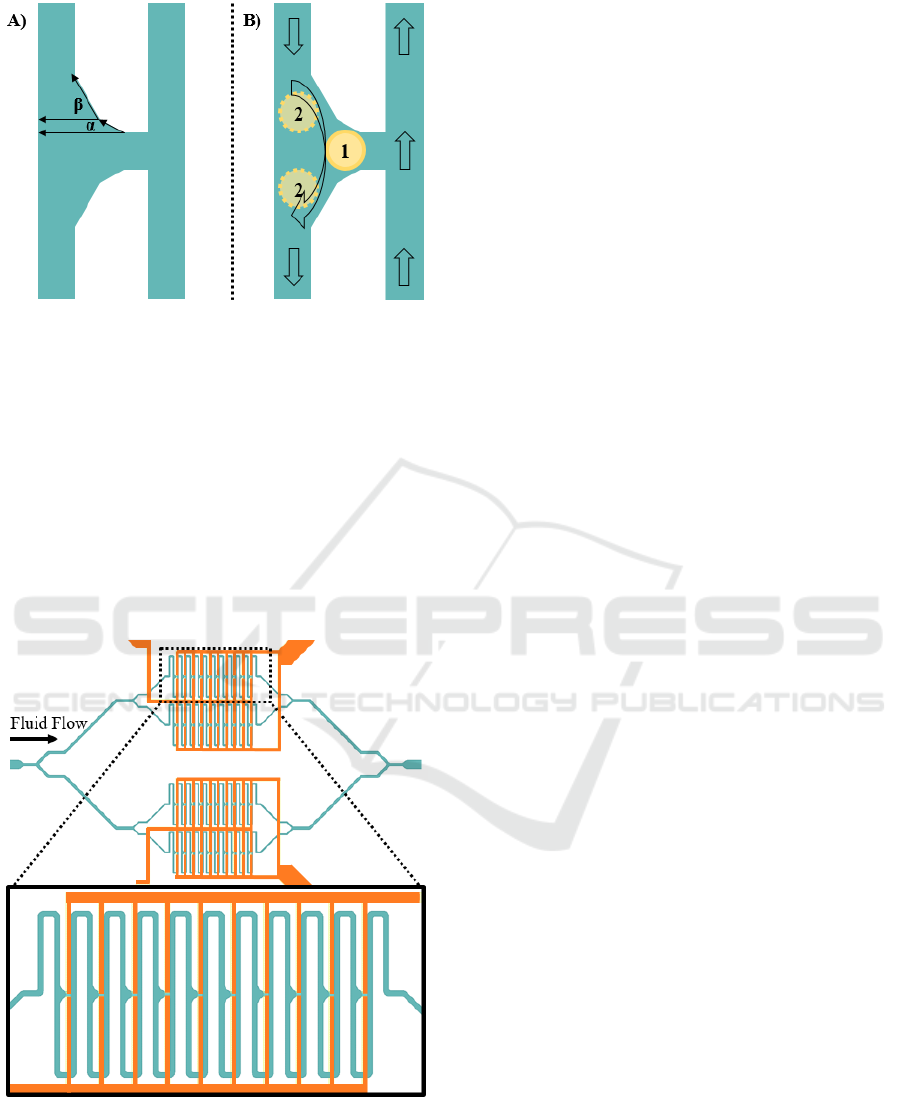
Figure 3: Schematic view of the graded triangular trap
design. A) α=30°, β=60° B) When a cell is trapped in
narrow region subsequent cells are cleared out of the trap
site.
Interdigitated titanium electrode couples are
placed under each trap site. The width of the
microelectrode fingers is 15µm and the gap between
the fingers is 8µm. Schematic view of the whole
system is given in Figure 4. There are 40 triangular
trap sites in total divided in four parallel lines of 10
trap sites.
The signal is recorded for 2 of the 4
parallel lines at the same time by interdigitated
electrode array couples connected to an LCR meter.
Figure 4: Schematic view of the whole trapping area.
There are 40 triangular trap sites in total. Green lines:
microfluidic channel, orange lines: interdigitated
microelectrodes.
3 MATERIALS AND METHODS
Conventional optical lithography processes are used
to create 3D microchannel structures by aligning two
optical mask to create a master mold for PDMS
casting. SU-8 3010 negative photoresist is used for
both layers. First layer has a thickness of 5µm and
the second layer is 10µm. Path 1 has a continuous
opening (transparent) in the first mask only to create
a vertical narrow neck in trap sites.
Titanium microelectrodes with a thickness of
200nm are fabricated on glass substrate coated with
a 400nm AZ 1505 positive photoresist by DC
magnetron sputtering and following lift-off process.
PDMS microchannel and microelectrodes coated
glass slide are aligned under an optical microscope
and plasma activated bonding process applied. A
droplet of methanol is used to create a sliding layer
between the PDMS microchannel and glass slide
during the alignment. Aligned substrates are placed
in a vacuum oven at 50˚C for 15 minutes to
evaporate the methanol and a stable bonding is
achieved.
MDA-MB-231 (human breast cancer cell line)
and HeLa cells (cervical cancer cell line) with
different medium conductivities are used in our
experiments. Conductivity of medium is adjusted by
the concentration of PBS (phosphate-buffered
saline) in 200mM sucrose solution (1X PBS=15,84
mS/cm, 0.5X PBS=7,71mS/cm).
4 EXPERIMENTS & DISCUSSION
Fluid flow is controlled by a syringe pump to
achieve precise flow rates and experiment is
observed under an optical microscope. Agilent
E4980A Precision LCR Meter (Keysight
Technologies, USA) is used for the impedance
measurements. Impedance values are recorded for a
frequency range of 1-500kHz with an applied
potential of 1V
pp
.
MDA-MB-231 and HeLa cells are efficiently
trapped in triangular sites and the signals are
recorded before and after the cells are trapped. Due
to the elastic nature of biological cells, some of them
are deformed through the bottom neck and slip
away. While the hydrodynamic trap design itself is
independent from the flow rate, it has been seen that
the design is most effective for flow rates below
2µl/min and cells with a minimum diameter of
10µm.
Single Cell Array Impedance Analysis for Cell Detection and Classification in a Microfluidic Device
51

MDA-MB-231 cells are trapped as seen in
Figure 5. Yellow circles represent empty traps and
green ones for filled traps with a single cell. The
impedance shift is recorded with the cells in green
circles.
Figure 5: MDA-MB-231 cells in PBS. Graded triangular
traps are used. The cells with no contact with
microelectrodes or empty traps are shown in yellow circles
where the cells are in contact with the microelectrodes are
shown in green. Each trap site has a single MDA-MB-231
cell.
A detailed view of filled and empty trap sites is
given in Figure 6.
Figure 6: Single cell trap sites: A) filled traps with a single
cell B) empty traps.
The impedance readings are recorded
continuously before and after releasing the cells into
the microchannel. When a cell is trapped the
impedance is shifted depending on the cell
properties. It can be seen that the impedance shift
(∆Z ) varies for each cell line which can be used for
further analysis or diagnosis applications. ∆Z values
for MDA-MB-231 and HeLa cells in 1XPBS are
given in Figure 7, showing larger shifts for MDA-
MB-231 cells compare to HeLa cells. This result
further proved that the impedance measurements are
sensitive to cell types.
Figure 7: Impedance shift of MDA-MB-231 and HeLa cell
lines in 1XPBS medium.
∆Z values for MDA-MB-231 cells in 1XPBS and
0.5XPBS mediums are given in Figure 8. It is found
that the magnitude of ∆Z increases with decreasing
conductivity of medium. This result shows that
medium conductivity can be adjusted to achieve
higher sensitivity for a target cell line.
Figure 8: Impedance shift of MDA-MB-231 cell lines in
0.5XPBS and 1XPBS mediums.
0
2
4
6
8
10
12
1 10 100 1000
∆Z(kΩ)
freq (kHz) (log)
HeLa MDA-MB-231
0
4
8
12
16
20
1 10 100 1000
∆Z(kΩ)
freq (kHz) (log)
MDA-MB-231 in 1XPBS
MDA-MB-231 in 0.5XPBS
BIODEVICES 2017 - 10th International Conference on Biomedical Electronics and Devices
52

The signal is collected from the array of 20
single cells in our current design but it is proven to
maintain these target cells in these trap sites
throughout the impedance analysis. As a future work
of our research, individual signals will be collected
from individual interdigitated electrode couples
placed under each trap sites to gain the long-term
data of impedance analysis of individual cells.
There is a recent study also inspired by Tan’s
design (Zhou et al., 2016) that presents
hydrodynamic trapping and impedance spectroscopy
of single cells. It has been shown that with a similar
design of our own, it is possible to monitor dynamic
changes in electrical properties of individual cells
over long periods of time to investigate the external
effects on cells.
5 CONCLUSIONS
Our experimental results show that diagnosing of
different cell lines in mediums with an optimum
conductivity is achievable using current single cell
trap array. The impedance shift is sensitive to cell
type and it can be used for the estimation of the total
number of captured target cells. The cell would be
stimulated by different chemicals or drugs injected
to microsystem to see the effects on cell viability or
its electrical properties. Further studies will focus on
introducing the optimum medium conductivity and a
frequency value for a target cell line to record the
impedance shift with a minimum error. This
technique will be used for estimating the
physical/electrical properties of cell structures and
the separation efficiency by DEP will be increased
with gained knowledge of target cell lines.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the financial
support provided by the Scientific and
Technological Research Council of Turkey
(TUBITAK) under Grant No. 114M802.
REFERENCES
Mateo, J., Gerlinger, M., Rodrigues, D., de Bono, J. S.,
2014. The promise of circulating tumor cell analysis in
cancer management. Genome Biol, 15(8), 448.
Karabacak, N. M., Spuhler, P. S., Fachin, F., Lim, E. J.,
Pai, V., Ozkumur, E., Toner, M., 2014. Microfluidic,
marker-free isolation of circulating tumor cells from
blood samples. Nature protocols, 9(3), 694-710.
Alix-Panabières, C., Pantel, K., 2014. Technologies for
detection of circulating tumor cells: facts and vision.
Lab on a Chip, 14(1), 57-62
Jin, C., McFaul, S. M., Duffy, S. P., Deng, X., Tavassoli,
P., Black, P. C., Ma, H., 2014. Technologies for label-
free separation of circulating tumor cells: from
historical foundations to recent developments. Lab on
a Chip, 14(1), 32-44.
Hajba, L., Guttman, A., 2014. Circulating tumor-cell
detection and capture using microfluidic devices.
TrAC Trends in Analytical Chemistry, 59, 9-16.
Park, H., Kim, D., Yun, K.S., 2010. Single-cell
manipulation on microfluidic chip by dielectrophoretic
actuation and impedance detection. Sensors and
Actuators B: Chemical, 150(1), 167-173.
Anh-Nguyen, T., Tiberius, B., Pliquett, U., & Urban, G.
A., 2016. An impedance biosensor for monitoring
cancer cell attachment, spreading and drug-induced
apoptosis. Sensors and Actuators A: Physical, 241,
231-237.
Dastider, S. G., Barizuddin, S., Yuksek, N., Dweik, M., &
Almasri, M., 2016. Impedance biosensor for rapid
detection of low concentration of E. coli 0157: H7.
In 2016 IEEE 29th International Conference on Micro
Electro Mechanical Systems (MEMS) (pp. 302-306).
IEEE.
Tan, W.H., Takeuchi, S., 2007. A trap-and-release
integrated microfluidic system for dynamic microarray
applications. Proceedings of the National Academy of
Sciences, 104(4), 1146-1151.
Zhou, Y., Basu, S., Laue, E., & Seshia, A. A., 2016.
Single cell studies of mouse embryonic stem cell
(mESC) differentiation by electrical impedance
measurements in a microfluidic device. Biosensors
and Bioelectronics, 81, 249-258.
Single Cell Array Impedance Analysis for Cell Detection and Classification in a Microfluidic Device
53
