
Synthesis of Pd@ZnO Core-shell Nanoparticles with Different Size
and Their Gas Sensing Properties
Yeon-Tae Yu
1
, Sanjit Manohar Majhi
1
, Gautam K. Naik
1
and Ho-Geum Song
2
1
Division of Advanced Materials Engineering and Research Centre for Advanced Materials Development,
College of Engineering, Chonbuk National University, Jeonju, 54896, Republic of Korea
2
Ogam Technology, Jeonju, 54882, Republic of Korea
Keywords: Gas Sensor, Palladium, ZnO, Core-shell, Nanoparticles, Response.
Abstract: Two different sizes of Pd@ZnO core-shell nanoparticles (NPs) have been prepared by using two different
sizes of Pd NPs (15 and 50 nm) as metal cores and applied for acetaldehyde gas sensing. Transmission
electron microscopy images revealed that the overall size of two sensing materials such as Pd
15
@ZnO and
Pd
50
@ZnO core-shell NPs are 80-100 nm and 100-120 nm, respectively. Xray-diffraction pattern revealed
that the oxidation of Pd metal core was started from 300C. The spherical shape and size are maintained
after the Pd@ZnO core-shell NPs was calcined at 500C for 2 h. PdO
15
@ZnO core-shell NPs showed
higher response to acetaldehyde. The maximum response of PdO
15
@ZnO core-shell NPs to 100 ppm of
acetaldehyde at 350 C was 75, whereas the maximum response of PdO
50
@ZnO core-shell NPs to 100 ppm
of acetaldehyde was 28 as compared to the pure ZnO NPs (Rs=18). The high response of PdO
15
@ZnO core-
shell NPs than PdO
50
@ZnO core-shell NPs is due to the smaller size of PdO core, which has more catalytic
activity than 50 nm sized PdO core.
1 INTRODUCTION
The advancement in science and technology has
resulted in rapid growth of urbanization,
industrialization and automobiles. The dark side of
the rapid growth in these sectors is the release of
many toxic gaseous pollutants in the environment
which are not only causing environmental pollution
but also serious health problems. The sever
condition of air pollution due to toxic and flammable
gases resulted in rapid growth of gas sensor
technology (Balouria, 2013). Among various gas
sensors, metal oxide semiconductor (MOS) gas
sensors are believed to be the best sensing materials
so far owing to their simple sensing mechanism, low
cost and ability to detect number of gases
(Korotcenkov, 2007). However, there are certain
issues, such as low sensitivity, poor selectivity and
high operating temperature, which needs to be
overcome for further advancement of the MOS
based gas sensors. Therefore, syntheses of highly
sensitive and selective gas sensing materials have
received much attention from the researchers
worldwide. Many approaches have been started for
the enhancement of gas sensing performance in
terms of sensitivity and selectivity, by aliovalent
doping, functionalization of sensing materials with
noble metals or creating metal oxide based
heterostructures. Recently, noble metal nanoparticles
(NPs) such as Au, Pd and Ag have been widely used
in gas sensing applications due to their catalytic
properties (Li, 2015). Among different ways, one of
the ways to utilize these noble metal NPs in gas
sensor is the design of core-shell hybrid structures,
where the noble metal in contact with the oxide
semiconductor plays a great role to enhance the
sensor performance in terms of sensitivity and
selectivity. In the core-shell structure, the core is
isolated from the shell, and prevents it from
aggregation during sintering. (Majhi, 2015). It is
known that the structure and morphology of sensing
material affects the sensor performances. However,
it is also known that the shape and size of noble
metal NPs also tailor the properties of a catalyst.
Hence, the optimization of size of noble metal NPs
along with the structure and morphology of metal
core-oxide shell is very important in gas sensing
field. (Rai, 2013) Among different noble metal NPs,
Pd is an important catalyst used in various
applications such as photocatalysis, alcohol
Yu Y., Majhi S., Naik G. and Song H.
Synthesis of Pd@ZnO Core-shell Nanoparticles with Different Size and Their Gas Sensing Properties.
DOI: 10.5220/0006273102070211
In Proceedings of the 6th International Conference on Sensor Networks (SENSORNETS 2017), pages 207-211
ISBN: 421065/17
Copyright
c
2017 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
207

oxidation reaction, CO oxidation, including gas
sensing such as alcohol, acetaldehyde and H
2
. Zinc
oxide (ZnO) with a band gap of 3.37 eV, high
excitation binding energy of 60 mV, high mobility
of conduction electron (200 cm
2
/ (V s)), has
exhibited promising role in many potential
applications including gas sensors. (Majhi, 2015)
Here in, we report a facile synthesis of Pd@ZnO
core-shell NPs with two different sizes by using two
different size of Pd metal NPs (15 and 50 nm) as
core material for gas sensing application. It is known
that Pd metal can be easily oxidised to PdO.
Therefore, in this work we want to investigate the
oxidation behaviour of Pd metal core of Pd@ZnO
core-shell NPs. The gas sensing study for
acetaldehyde gas will be carried out for two different
sizes of PdO@ZnO core-shell NPs. The details of
the synthesis procedure, gas sensing properties and
mechanism will be discussed in this report.
2 EXPERIMENTAL
2.1 Synthesis of 15 nm Sized Pd NPs
To synthesize Pd nanoparticles (NPs), initially 2.5
ml of H
2
PdCl
4
(10 mM) was added to 50 mL of
CTAB aqueous solution (12.5 mM) and heated at
100C with rapid stirring. Then, 400 L of freshly
prepared AA (0.1 M) was quickly added to the
above solution and the stirring continued for about 5
min to produce ~ 15 nm Pd NPs.
2.2 Synthesis of 50 nm Sized Pd NPs
Initially 12 ml of 0.001 M H
2
PdCl
4
was taken in a
beaker. To this 3ml of 0.01M Tri-sodium citrate
dihydrate was dropped and stirred for three minute.
To the above solution 3 ml of 0.01M ascorbic acid
(AA) was dropped with stirring. The reaction was
further continued for 30 min for complete reduction.
2.3 Synthesis of Pd
15
@ZnO and
Pd
50
@ZnO Core-shell NPs
Both the core-shell NPs were carried out in separate
vessels using two different sizes of Pd NPs.
Typically, a certain amount of CTAB were added
into 60mL of DI water and kept at 60 C in oven for
five minutes for proper dissolving. After that, a
certain amount of AA was added with stirring. To
the above solution, calculated amounts of ZnNO
3
and HMTA were added and stirred well. Then, 3 mL
of both 15 and 50 nm sized Pd NPs were dropped to
the above solution with stirring. Finally, the above
solution was transferred and heated in oven at 85
o
C
for 8h without stirring. After the reaction completed,
the products were centrifuged, washed carefully for
several times and then dried at 60
o
C in oven. The
final products of Pd@ZnO core-shell NPs obtained
were calcined at 500
o
C for 2 h. The synthesis of pure
ZnO NPs was also carried out using similar method
except adding of Pd NPs.
2.4 Characterizations
The as prepared products were analyzed by TEM
(Hitachi-H7650), selected area electron diffraction
(SAED) pattern, high-angle annular dark-field
scanning TEM (HAADF-STEM), high resolution
real-time line scan mapping and HRTEM (HRTEM,
Zeiss EM-912, Omega). The powder XRD for as
prepared products was analyzed by D/Max-2005,
Rigaku, X-ray diffractometer (Cu K
α,
λ =1.54178
Å).
2.5 Gas Sensor Device Fabrication and
Testing
About 10 mg of as prepared sensing materials were
mixed and grounded evenly with α-terpineol to
make a paste, which was coated on the surface of
alumina substrates (area:15 mm × 15 mm) and then
dried at 60
o
C. The as obtained sensor devices were
heat-treated 500
o
C for 2 h in air for stabilization
and to remove the solvent, before the gas sensing
test. Both the sensor devices were tested under a
temperature-controlled environment. The test
temperature was 350
o
C and acetaldehyde was used
as target gas. Nitrogen gas was used as background
gas and the dry air was mixed to be 10.5% of
oxygen. The change in resistance of the device due
to the presence or absence of test gas was measured
using a resistance meter (Agilent 34970A). The
sensor response (R
s
) was calculated using (R
a
/R
g
)
where R
a
is the resistance in air, and R
g
is the
resistance measured during the exposure of target
gas.
SENSORNETS 2017 - 6th International Conference on Sensor Networks
208
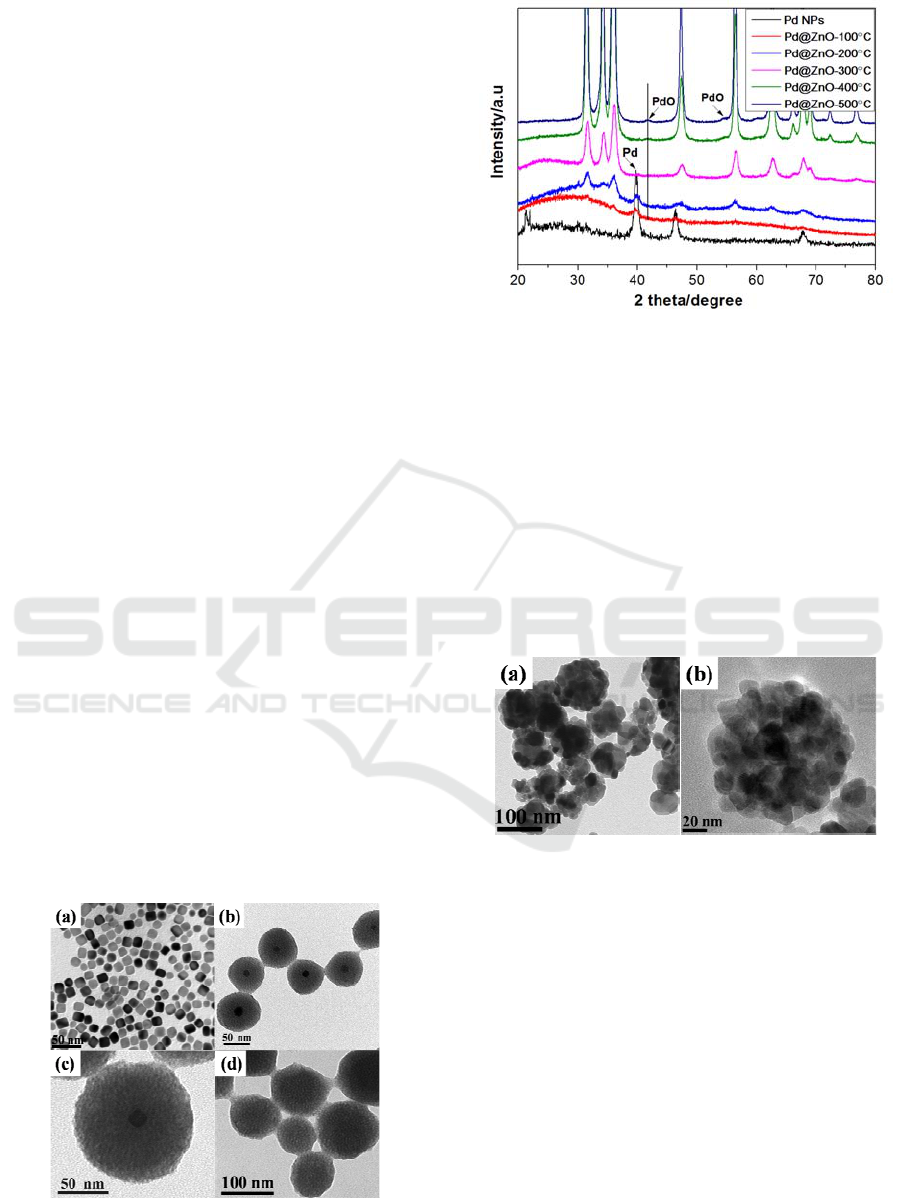
3 RESULTS AND DISCUSSION
3.1 Morphology and Structural
Characterization of Pd
15
@ZnO
Core-shell NPs
Figure 1a is the TEM image of CTAB assisted Pd
NPs, which shows the formation of mixed type of
shapes, such as semi-cubic and spherical. The size of
Pd NPs was in the range of 5 to 15 nm. Figure 1b is
the TEM image of as synthesized Pd
15
@ZnO core-
shell NPs. The size of spherical shaped Pd
15
@ZnO
core-shell NPs was in the range of 70-100 nm and
each Pd@ZnO core-shell particles contains only one
Pd NP in the centre of ZnO shell. Spherical shaped
ZnO NPs in the range of 70-120 nm were formed in
the absence of Pd NPs shows in Figure 1d.
Generally, the gas sensor device fabricated requires
heat-treatment at elevated temperature before gas
sensing measurement and in our study the device
was activated at 500
o
C. Therefore, the effect of heat-
treatment on the morphology and phase of
Pd
15
@ZnO core-shell was investigated. The phase
and structural analysis of Pd
15
@ZnO core-shell NPs
after the heat treatment was analyzed by XRD and
shown in Figure 2. Since Pd metal can be oxidised to
PdO at high temperatures in air, to know the
oxidation behaviour of Pd metal, Pd@ZnO core-
shell NPs were calcined at different temperatures
starting from 100 to 500C. Figure 2 shows the
diffraction patterns of pure Pd NPs (15 nm sized)
and Pd
15
@ZnO core-shell NPs calcined at different
temperatures from 100 to 500C-2 h. The diffraction
pattern for Pd
15
@ZnO core-shell NPs can be index
to wurtzite ZnO (JCPSD 36-1451). In case of Pd
NPs the strongest three diffraction peaks
corresponding to (111), (222) and (200) lattice
planes of face centered cubic (FCC) metallic Pd.
Figure 1: TEM images of the as synthesized (a) Pd NPs (b,
c) Pd
15
@ZnO core-shell and (d) pure ZnO NPs.
Figure 2: XRD patterns of the as synthesized Pd NPs, and
Pd@ZnO core-shell NPs calcined at different temperatures
from 100 to 500C for 2 h.
However, in case of Pd
15
@ZnO core-shell NPs, PdO
peaks are found from 300C calcination temperature
With the increasing the calcination temperature of
Pd
15
@ZnO core-shell NPs the intensity of PdO
peaks increases. Figure 3a and b depicts the TEM
images of pure ZnO and Pd
15
@ZnO core-shell NPs
after calcined at 500C for 2 h. The spherical shape
and size was maintained after the heat treatment at
500C for 2 h. However, the ZnO shell becomes
more crystalline after the calcination.
Figure 3: TEM images of (a) ZnO NPs and (b)
PdO
15
@ZnO core-shell NPs after calcined at 500C.
3.2 Morphology and Structural
Characterization of Pd
50
@ZnO
Core-shell NPs
To prepare the different sizes of Pd@ZnO core-shell
NPs, about 50 nm sized Pd NPs were synthesized by
sodium citrate method and then used to prepare
Pd
50
@ZnO core-shell NPs which was shown in
Figure 4. Figure 4a shows the sodium citrated
method synthesized around 50 nm sized Pd NPs,
which are nearly spherical in size. Figure 4b shows
the TEM image of Pd
50
@ZnO core-shell NPs and
the total size are around 110-120 nm. Figure 4c is
the TEM image of Pd
50
@ZnO core-shell NPs after
Synthesis of Pd@ZnO Core-shell Nanoparticles with Different Size and Their Gas Sensing Properties
209
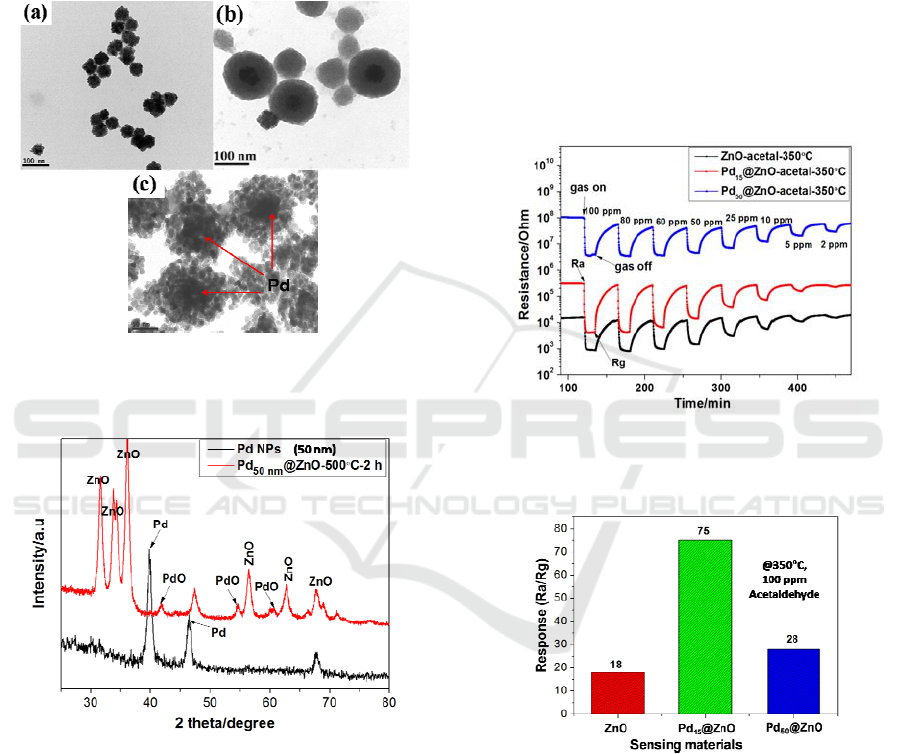
calcined at 500C for 2 h. The spherical shape and
size also maintained as like as Pd
15
@ZnO core-shell
NPs. The phase structure was analyzed by XRD
which is shown in Figure 5. The XRD patterns
indicates that after calcined at 500C for 2 h, the 50
nm sized Pd metal core in Pd
50
@ZnO core-shell NPs
also was oxidized to PdO.
Figure 4: TEM images of the as synthesized (a) 50 nm
sized Pd NPs, (b) Pd
50
@ZnO core-shell and (c)
PdO
50
@ZnO core-shell NPs calcined at 500C for 2 h.
Figure 5: XRD patterns of 50 nm sized Pd NPs and
PdO
50
@ZnO core-shell NPs after calcined at 500C.
3.3 Gas Sensing Properties
To know the gas sensing performance, all the three
sensor devices such as pure ZnO, PdO
15
@ZnO and
PdO
50
@ZnO NPs were investigated for
acetaldehyde gas. Figure 6 shows the dynamic
response of all the three sensors when orderly
exposed to acetaldehyde from 2 to 100 ppm. Since
the Pd metal core was oxidised to PdO from 300C
calcination temperature, in this study the gas sensing
testing temperature was kept at 350C. It can be seen
that the corresponding responses of sensors were
dependent on the concentration of acetaldehyde.
With increasing the acetaldehyde concentration the
response increases. It can be seen that all the three
sensors show the typical n-type semiconducting
behaviour that is after the expose of target gas the
resistance decreases. The responses of all the three
sensors tested at 350C for 100 ppm of acetaldehyde
is shown in Figure 7. The maximum response of
PdO
15
@ZnO core-shell NPs for 100 ppm
acetaldehyde was 75 whereas the maximum
response of PdO
50
@ZnO core-shell NPs was 28 as
compared to the pure ZnO ( Rs=18).
Figure 6: Response transient of all three sensors such as
pure ZnO, PdO
15
@ZnO and PdO
50
@ZnO core-shell NPs
tested at 350C for acetaldehyde (2-100 ppm).
Figure 7: Response of all three sensing materials tested for
100 ppm of acetaldehyde at 350C.
3.4 Gas Sensing Mechanism
Figure 8 shows the gas sensing mechanism of pure
ZnO NPs and PdO@ZnO core-shell NPs in air and
acetaldehyde medium. The response of PdO@ZnO
core-shell NPs shows higher than bare ZnO NPs,
which is due to the more depletion layer formation
in PdO@ZnO core-shell NPs, which increases the
resistance of the core-shell NPs when exposed in air.
SENSORNETS 2017 - 6th International Conference on Sensor Networks
210

After the acetaldehyde gas was exposed, the gas
molecules react with oxygen ion species and releases
more number of electrons to the conduction band of
PdO@ZnO core-shell than pure ZnO. Hence the
response of PdO@ZnO core-shell NPs shows higher
than pure ZnO NPs. However, the high response of
PdO
15
@ZnO core-shell NPs than PdO
50
@ZnO core-
shell NPs is due to the smaller size of PdO core,
which has more catalytic activities than 50 nm sized
PdO core (Ma, 2015).
Figure 8: Gas sensing mechanism of (a) pure ZnO NPs
and (b) PdO@ZnO NPs in air and acetaldehyde gas
medium.
4 CONLUSIONS
In summary, two different sizes of Pd@ZnO core-
shell NPs were successfully synthesized by a facile
and lower temperature approach, where two
different sizes of Pd core such as 15 and 50 nm were
used. The overall particles size of Pd
15
@ZnO core-
shell NPs was about 80-100 nm, whereas the total
size of Pd
50
@ZnO core-shell was 100-120 nm. The
spherical shape and structure of as prepared two
Pd@ZnO core-shell NPs were maintained after
calcined at 500C for 2 h. The Pd metal core was
oxidized to PdO from 300C calcination temperature.
The maximum response of PdO
15
@ZnO core-shell
NPs for 100 ppm of acetaldehyde at 350C was 75,
whereas the maximum response of PdO
50
@ZnO
core-shell NPs was 28 as compared to pure ZnO
(R
s
=18).The response of PdO
15
@ZnO core-shell
NPs is higher than PdO
50
@ZnO core-shell NPs. The
possible reason is due to the smaller size of Pd core,
which has more catalytic activity than 50 nm sized
Pd core.
ACKNOWLEDGEMENTS
This paper was supported by 1) BK21 plus program
from the Ministry of Education and Human-
Resource Development, 2) National Research
Foundation grant funded by the Korea government
(MSIP) (BRL 2015042417, 2016R1A2B4014090)
and 3) Business for Cooperative R&D between
Industry, Academy, and Research Institute funded
Korea Small and Medium Business Administration
in 2016 (Grants No. C0396231).
REFERENCES
Balouria, V., Kumar, A., Samanta, S., Singh, A., Debnath,
AK., Mahajan, A., Bedi, RK., Aswal, DK., Gupta, SK.,
2013. Nanocrystalline Fe
2
O
3
thin films for ppm level
detection of H
2
S. Sens Actuators B Chem. 471-478.
Korotcenkov, G., 2007. Review: Metal Oxides For Solid-
State Gas Sensors: What Determines Our Choice?
Mater. Sci. Eng., B. 1-23.
Li, X., Liu, J., Guo, H., Zhou, X., Wang, C., Sun, P., Lu,
G., 2015. Au@In
2
O
3
core-shell composite: a metal-
semiconductor heterostructures for gas sensing
applications. RSC Adv. 545-551.
Ma, N., Suematsu, K., Yuasa, M., Shimanoe, K., 2015. Pd
size effect on the gas sensing properties of Pd-loaded
SnO
2
in humid atmosphere. ACS Appl. Mater.
Interfaces. 15618-15625.
Majhi, S.M., Rai, P., Yu, Y-T., 2015. Facile Approach to
Synthesize Au@ZnO Core-Shell Nanoparticles and
Their Application for Highly Sensitive and Selective
Gas Sensor. ACS Appl. Mater. Interfaces. 9462-9468.
Majhi, S.M., Rai, P., Raj, S., Chon, B.S., Park, K-K., Yu,
Y-T., 2014. Effect of Au nanorods on potential barrier
modulation in morphologically controlled Au@Cu
2
O
core-shell nanoreactors for gas sensor applications.
ACS Appl. Mater. Interfaces.7491-7497.
Rai, P., Kwak, W-K., Yu, Y-T., 2013. Solvothermal
Synthesis of ZnO Nanostructures and Their
Morphology-Dependent Gas-Sensing Properties. ACS
Appl. Mater. Interfaces. 36-50.
Synthesis of Pd@ZnO Core-shell Nanoparticles with Different Size and Their Gas Sensing Properties
211
