
Models for Predicting the Development of Insulin Resistance
Thomas Forstner
1,*
, Christiane Dienhart
2
, Ludmilla Kedenko
2
, Gernot Wolkersdörfer
2
and Bernhard Paulweber
2
1
Department of Applied Systems Research and Statistics, Johannes Kepler University Linz,
Altenberger Strasse 69, 4040 Linz, Austria
2
Department of Internal Medicine I, Paracelsus Medical University/Salzburger Landeskliniken,
Muellner Hauptstrasse 48, 5020 Salzburg, Austria
Keywords: Predictive Models, Insulin Resistance, Logistic Regression, ROC Analysis.
Abstract: Insulin resistance is the leading cause for developing type 2 diabetes. Early determination of insulin
resistance and herewith of impending type 2 diabetes could help to establish sooner preventive measures or
even therapies. However, an optimal predictive model for developing insulin resistance has not been
established yet. Based on the data of an Austrian cohort study (SAPHIR study) various predictive models
were calculated and compared to each other. For developing predictive models logistic regression models
were used. For finding an optimal cut-off value an ROC approach was used. Based on various biochemical
parameters an overall percentage of around 82% correct classifications could be achieved.
1 INTRODUCTION
Insulin resistance (IR) is a condition in which,
muscle, fat and liver cells do not respond properly to
insulin and these cells therefore cannot easily absorb
glucose from the bloodstream. So, the body needs
higher levels of insulin to help glucose enter the
cells and as consequence the so called beta cells in
the pancreas subsequently increase their production
of insulin to try to keep up with this increased
demand for insulin by producing more insulin. As
long as the beta cells are able to produce enough
insulin to overcome the insulin resistance of the
cells, blood glucose levels stay in a healthy range.
But over time the beta cells fail to keep up with the
body's increased need for insulin and without
enough insulin, excess glucose builds up in the
bloodstream. This can lead to diabetes and other
serious health disorders (Rutter et al., 2008).
Diabetes is often asymptomatic and around 50%
of patients suffering from type 2 diabetes are
undiagnosed and the delay from disease onset to
diagnosis can exceed 10 years. At the time patients
are diagnosed around 25% have established
retinopathy and around 50% have signs of diabetic
tissue damage (Griffin et al., 2000). Furthermore
type 2 diabetes is affecting around 8% of the current
global adult population and the prevalence is
growing worldwide (Keating, 2015).
Early determination of insulin resistance and
herewith of impending type 2 diabetes could help to
establish sooner preventive measures or even
therapies.
The gold-standard for quantifying insulin
resistance is the hyperinsulinemic euglycemic clamp
but this impractical in epidemiological studies
because the measurement takes about two hours and
medical attention is needed. Therefore a commonly
used surrogate endpoint is the homeostatic model
assessment (HOMA) which is derived from the
product of fasting glucose and insulin levels.
The HOMA index is a robust tool for the
assessment of insulin resistance in epidemiological
studies. HOMA index values larger 2.0 are used as an
indication for insulin resistance (Griffin et al., 2000).
The aim of this work was to develop a predictive
model for insulin resistance based on various clinical
parameters and to evaluate this model based on an
ROC approach.
2 DATA
As study population the data of an Austrian cohort
study (SAPHIR study, Salzburg Atherosclerosis
Prevention program in subjects at High Individual
Risk) was used.
Forstner, T., Dienhart, C., Kedenko, L., Wolkersdörfer, G. and Paulweber, B.
Models for Predicting the Development of Insulin Resistance.
DOI: 10.5220/0006344801210125
In Proceedings of the 6th International Conference on Data Science, Technology and Applications (DATA 2017), pages 121-125
ISBN: 978-989-758-255-4
Copyright © 2017 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
121
The prospective SAPHIR study was conducted
from 1999 to 2002 and involved unrelated male and
female subjects between 40 and 70 years, who lived
in the greater Salzburg region and responded to
invitations by their family or workplace physician or
to announcement in the local press. The study has
been approved by the ethical committee of Salzburg
and written consent was granted by the participants.
At baseline and after approximately 3 years the
participants were subjected to a screening program
that included a personal and family history and a
physical examination. These evaluations also
included a panel of laboratory tests, measurement of
visceral fat mass, body composition, insulin
sensitivity, intima-media thickness of the carotid
arteries and a detailed medical history. HOMA
indices have been used to define the level of insulin
sensitivity.
In total, 1,770 subjects were included in the
SAPHIR study. All subjects of the SAPHIR study
were invited to a second follow-up examination 5
years after their completion of the SAPHIR study.
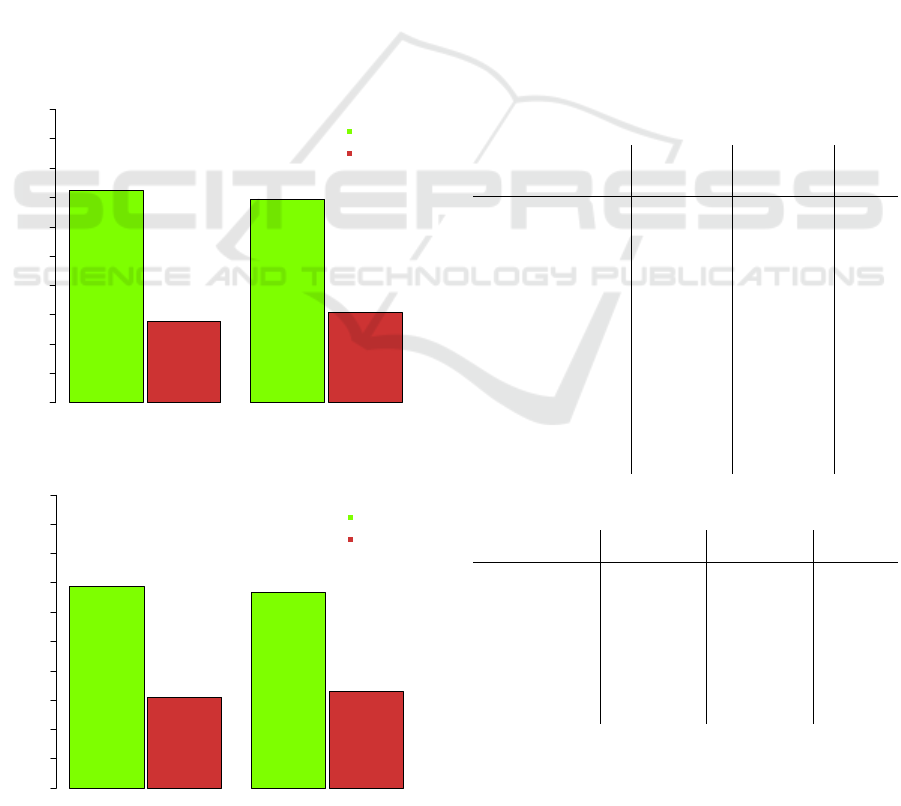
Figure 1: Insulin resistance, SAPHIR study population.
Figure 2: Insulin resistance, complete case population.
This resulted in a response of 1386 subjects,
which were used for the model building process.
Because of missing data only 480 (“complete case
population”) of these 1386 subjects could be used.
The distribution of insulin resistance was similar
between the two populations. The largest difference
was 3.4 percent points. Subjects with insulin
resistance at baseline were also included into the
model because in “real life” the status of insulin
resistance of the subject at baseline is unknown, so
exclusion would lead to a potential bias
3 PREDICTIVE MODELS
3.1 Approach
Because of the binary nature of the data a logistic
regression approach was chosen to predict the target
variable “indication of insulin resistance at follow-
up”. The baseline covariates there chosen from a
clinical point of view.
Table 1: Continuous baseline covariates.
Baseline covariates
No IR
(mean ± SD)
IR
(mean ± SD)
p-value
2hr-OGTT glucose level
[ /dl]
32.38 ± 28.98 81.47 ± 64.68 <0.001
**
Adiponectin [µg/ml]
9.19 ± 4.74 6.94 ± 3.67 <0.001
**
Age [years]
51.06 ± 6.08 52.26 ± 5.72 0.001
**
BMI [kg/m²]
25.55 ± 3.33 30.09 ± 4.20 <0.001
**
Fat mass [kg]
16.82 ± 7.8 25.13 ± 9.26 <0.001
**
F-Insulin [µU/ml]
5.14 ± 1.82 13.53 ± 5.98 <0.001
**
Glucose [mg/dl]
89.17 ± 8.79 104.91 ± 27.71 <0.001
**
HbA1c [%]
5.53 ± 0.30 5.87 ± 0.86 <0.001
**
HDL cholesterol [mg/dl]
62.73 ± 15.71 51.43 ± 12.37 <0.001
**
HOMA-Index
1.14 ± 0.42 3.53 ± 1.97 <0.001
**
kITT-Index
4.51 ± 1.20 3.24 ± 1.17 <0.001
**
Lean mass [kg]
59.35 ± 11.65 64 ± 12.96 <0.001
**
Triglyceride [mmol/]
108.7 ± 74.21 168.27 ± 102.39 <0.001
**
Waist-Hip-Ratio
0.88 ± 0.08 0.93 ± 0.07 <0.001
**
Table 2: Categorical baseline covariates.
Baseline covariates
No IR
(%, n)
IR
(%, n)
p-value
Gender
female
male
476 (72.8%)
795 (72.1%)
178 (27.2%)
307 (27.9%)
0.783
Hypertension
no
yes
789 (80.8%)
457 (62.0%)
188 (19.2%)
280 (38.0%)
<0.001
**
Incident diabetes
no
yes
1261 (75.0%)
10 (13.5%)
421 (25.0%)
64 (86.5%)
<0.001
**
Hypertension was defined as a systolic blood
pressure above 130 mmHg and a diastolic blood
pressure above 85 mmHg. In the early stages of
diabetes sometimes no insulin resistance has
Insulin Resistance - SAHPIR Study Population
Valid %
0 102030405060708090100
72.4% (n=1271)
27.6% (n=485)
69.4% (n=946)
30.6% (n=418)
NO
YES
Baseline Follow-Up
Insulin Resistance - Complete Case Population
Valid %
0 102030405060708090100
69.0% (n=331)
31.0% (n=149)
66.9% (n=321)
33.1% (n=159)
NO
YES
Baseline Follow-Up
DATA 2017 - 6th International Conference on Data Science, Technology and Applications
122

developed yet, therefore baseline incident diabetes
was also included as a covariate in the model.
Incident diabetes was defined as a fasting plasma
glucose level ≥ 126 mg/dl, a 2-hr OGTT glucose of
≥ 200 mg/dl or current use of hypoglycaemic drug
therapy regardless the reason.
Continuous data was compared between the two
groups using the two sample independent t-test in
case of normality (verification with the
Kolmogorov-Smirnov test with Lilliefors correction)
and variance homogeneity (verification with the
Levene test). If continuous data was normally
distributed but variance was heterogeneous Welch’s
t-test was used and if data showed no normal
distribution, the Mann-Whitney-U test was used.
Unpaired categorical data was compared using
Fisher´s exact test. For calculations SPSS (IBM
Corp. Released 2013. IBM SPSS Statistics for
Windows, Version 22.0. Armonk, NY: IBM Corp.)
and the statistical computing software R Version
3.2.3 (R Foundation for Statistical Computing,
Vienna, Austria. URL http://www.R-project.org)
were used. The uncorrected type I error was set at
5% (two-sided), this means no adjustment for the
type I error was made.
3.2 First Model
The a-priori selected baseline covariates (except
gender) discriminated very well regarding the
indication of baseline insulin resistance, so for a first
model all baseline covariates were used to predict
indication of insulin resistance at follow-up.
The estimates based on this logistic regression
model (model Ia) are presented in the table below.
Table 3: Variables of the model Ia.
Baseline Covariates p-value Odds-Ratio 95% CI for OR
2hr-OGTT glucose level [mg/dl] 0.001
**
1.015 1.006-1.024
Adiponectin [µg/ml] 0.038
*
0.911 0.834-0.995
Age [years] 0.547 0.983 0.931-1.039
BMI [kg/m²] 0.077 1.169 0.983-1.39
Fat mass [kg] 0.407 0.972 0.91-1.039
F-Insulin [µU/ml] 0.001
**
1.706 1.24-2.348
Glucose [mg/dl] 0.011
*
1.058 1.013-1.106
HbA1c [%] 0.977 0.986 0.397-2.452
HDL cholesterol [mg/dl] 0.010
*
0.963 0.936-0.991
HOMA-Index 0.008
**
0.212 0.067-0.672
kITT-Index 0.893 1.017 0.794-1.303
Lean mass [kg] 0.980 1.000 0.951-1.051
Triglyceride [mmol/] 0.979 1.000 0.996-1.004
Waist-Hip-Ratio 0.394 9.645 0.052-1773.282
Gender (male vs. female) 0.037
*
3.886 1.086-13.897
Hypertension (no vs. yes) 0.395 0.776 0.432-1.393
Incident diabetes (no vs. yes) 0.708 1.403 0.238-8.265
Constant 0.004
**
0.000 1.006-1.024
This model yielded an overall percentage of
82.2% correct classifications (90.5% correct
classifications for no indication of insulin resistance
and 65.3% correct classifications for indication of
insulin resistance). A Nagelkerke’s R² of 0.550
indicated also a good model fit.
Based on the model an increase in one unit of
“2hr-OGTT glucose level [mg/dl]”, “F-Insulin
[µU/ml]” and “Glucose [mg/dl]” is associated with a
significant higher chance for insulin resistance. An
increase of one unit in “Adiponectin [µg/ml]” and
“HDL cholesterol [mg/dl]” is associated with a
significant lower chance for insulin resistance. There
was also a significant increased chance for women
suffering from insulin resistance at follow-up.
Interestingly low levels of HOMA-index
baseline were associated with a higher chance of
insulin resistance at follow-up (Odds Ratio = 0.212).
But a univariate analysis yielded the expected
association (Odds Ratio = 4.315, p < 0.001**). So
this unexpected multivariate Odds Ratio may be
caused through the multicollinearity of the model.
The confidence interval of the “Waist-Hip-
Ratio” was extremely large so a bootstrapping
approach based on 1000 bootstrap-samples was used
to validate the numerical integrity of model Ia. The
confidence interval for “Waist-Hip-Ratio” was even
more extreme so this variable was excluded from the
model. The results from this model (model Ib)
remained almost the same like model Ia.
The classification results of the model Ib are
presented in the table below.
Table 4: Classification result of model Ib.
Predicted
Observed
no IR IR
% correct
no IR
272 29 90.4
IR
51 97 65.5
Overall %
82.2
(Cut-Off value 0.5)
3.3 Second Model
The first model yielded a good overall percentage of
correct classification results but was relatively
complex because various laboratory parameters were
needed. So the next step was to reduce the
complexity of the model using a variable selection
approach (Bursac et al., 2008). A reduced model
with similar results but fewer needed laboratory
parameters would also be more cost efficient.
The first approach was using a backwards-
likelihood-ratio-approach (probability for entry 0.05,
probability for removal 0.10) which led to an overall
percentage of correct classification of 81.7% but this
Models for Predicting the Development of Insulin Resistance
123
model was again relatively complex (8 covariates
were selected). So the next step was using a
forwards-likelihood-ratio-approach (probability for
entry 0.05, probability for removal 0.10) which
resulted in a similar overall percentage of correct
classifications but with a reduced number of
covariates.
The estimates based on this forward-logistic
regression model (model II) are presented in the
table below.
Table 5: Variables of the model II.
Baseline Covariates p-value Odds-Ratio 95% CI for OR
2hr-OGTT glucose level [mg/dl] 0.001
**
1.013 1.005-1.021
Adiponectin [µg/ml] 0.012
*
0.900 0.83-0.977
BMI [kg/m²] 0.002
**
1.123 1.044-1.207
F-Insulin [µU/ml] <0.001
**
1.188 1.095-1.289
HDL cholesterol [mg/dl] 0.001
**
0.959 0.936-0.982
Gender (male vs. female) 0.005
**
2.599 1.337-5.052
Constant 0.001
**
0.019 1.005-1.021
The result was a relatively compact model with an
overall percentage of 81.9% correct classifications
(91.6% correct classifications for no indication of
insulin resistance and 62.3% correct classifications for
indication of insulin resistance). A Nagelkerke’s R² of
0.531 indicated again a good model fit.
Based on this model an increase in one unit of
“2hr-OGTT glucose level [mg/dl]”, “BMI [kg/m²]”
and one unit of “F-Insulin [µU/ml]” is associated with
a significant higher chance for insulin resistance.
An increase in one unit of “Adiponectin
[µg/ml]” and one unit of “HDL cholesterol [mg/dl]”
is associated with a significant lower chance for
insulin resistance. There was also a significant
increased chance for women suffering from insulin
resistance at follow-up.
The robustness of this model was again verified
with a bootstrapping approach based on 1000
bootstrap-samples; no inconsistency was detected.
Compared to the overall percentage of correct
classifications and correct classifications for no
indication of insulin resistance the percentage of
correct classifications for indication of insulin
resistance was low. So based on a Receiver
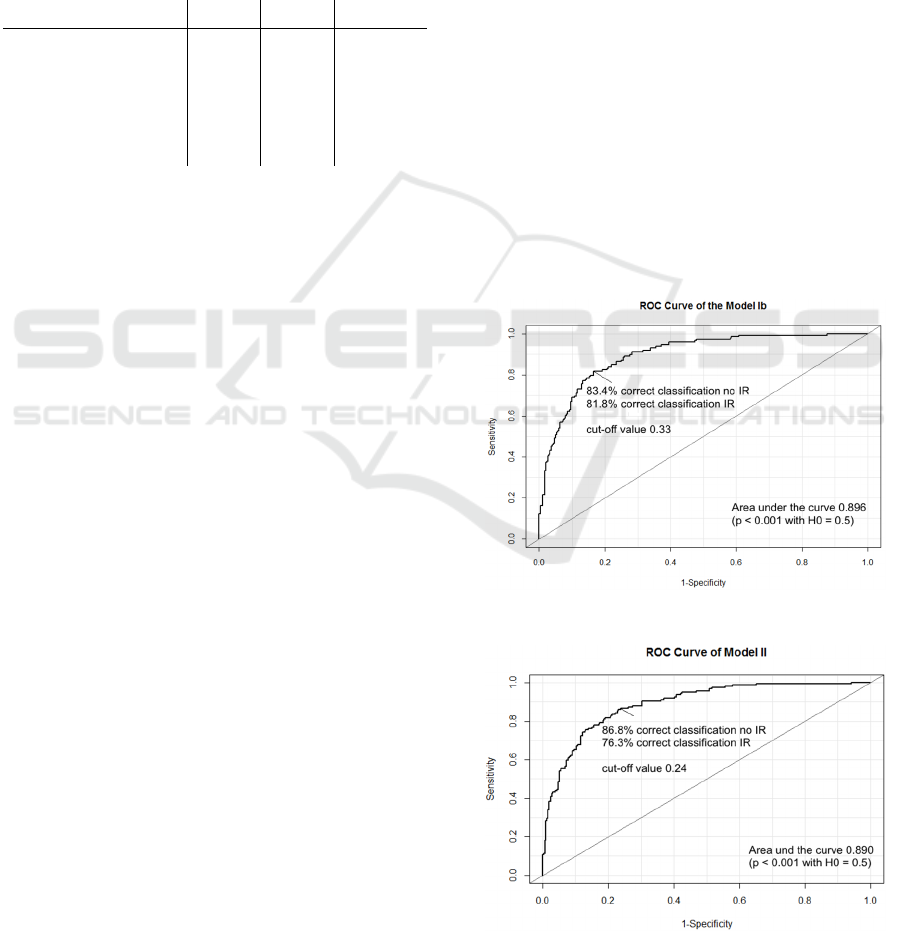
Operating Characteristic curve (ROC curve) an
optimal cut-off value for the predicted probabilities
was searched.
4 ROC ANALYSIS
A standard technique for summarizing the
performance of a predictive model over a range of
cut-off-values is the Receiver Operating
Characteristic curve (ROC curve) (Sweets, 1988).
Since the ROC curve summarizes the predictive
power of a model over all possible cut-off-values, it
is more informative than a simple classification table
for a fixed cut-off value.
The area under the curve (AUC) can be used as a
performance metric for ROC curves. A higher area
under the curve indicates a better prediction power.
The previous logistic regression models yielded an
area under the curve of 0.896 (model Ib)
respectively 0.890 (model II).
For the previous logistic regression models the
standard cut-off-value of 0.5 was used for the
predicted probabilities. To maximize the
effectiveness of a predictive model the cut-off value
can be varied to achieve a higher true positive rate
(“sensitivity”) and a higher true negative rate
(“specificity”). The Youden index method (Youden,
1950) was used to the define the optimal cut-off
value for the two models.
The idea of the Youden index is to maximize the
difference between true positive rate and false
positive rate (“1 – specificity”). This means in other
words that the Youden index is specified as the point
Figure 3: ROC curve for model Ib.
Figure 4: ROC curve for model II.
DATA 2017 - 6th International Conference on Data Science, Technology and Applications
124

on the ROC curve which has the largest distance
from the diagonal line. Based on the Youden index
an optimal cut-off-value of 0.33 (model Ib)
respectively 0.24 (model II) could be found.
In other papers an achieved area under the curve
of 0.71 (Chiang et al., 2011) or 0.75 (Behboudi-
Gandevani et al., 2016) was reported. In (Er et al.,
2016) various models for predicting insulin
resistance with an achieved area under the curve
from 0.31 up to 0.80 were reported. So the area
under the curve of the two presented predictive
models indicates a slightly better performance.
5 CONCLUSIONS
Based on the data of an Austrian cohort study a
predictive model with an overall percentage of
81.9% correct classifications for predicting insulin
resistance based on gender and 5 biochemical
parameters could be developed. Furthermore the
classification cut-off-value was optimized by the use
of ROC curves to achieve 86.6% correct
classifications for no indication of insulin resistance
and 76.3% correct classifications for indication of
insulin resistance. Additionally based on the R
package “caret” (Kuhn, 2008) a random forest
approach was used for predicting insulin resistance.
This approach yielded a similar overall percentage
(79.2%) of correct classifications (86.6% correct
classifications for no indication of insulin resistance
and 64.2% correct classifications for indication of
insulin resistance).
Limitations of this model are that the data was
collected in a relatively small area and that because
of various missing values in the baseline covariates
and target variable only around 480 of 1386 patients
could be included in the model building process. To
account for this relatively large percentage of
missing values a multiple imputation approach (5
imputations, fully conditional specification method
based on an iterative Markov chain Monte Carlo
algorithm) was used. The results of this approach
yielded very similar results except that the influence
of the covariate gender was not significant anymore
(Odds Ratio = 0.997; p = 0.989) like the univariate
test of gender between baseline insulin resistance.
REFERENCES
Behboudi-Gandevani S., Tehrani F. R., Cheraghi L., Azizi
F., 2016. Could “a body shape index” and “waist to
height ratio” predict insulin resistance and metabolic
syndrome in polycystic ovary syndrome. European
Journal of Obstetrics & Gynecology and Reproductive
Biology, 205: 110-114.
Bursac Z., Gauss C. H., Williams D. K., Hosmer D. W.,
2008. Purposeful selection of variables in logistic
regression. Source Code for Biology and Medicine,
17(3).
Chiang J. K., Lai N. S., Chang J. K., Koo M., 2011.
Predicting insulin resistance using the triglyceride-to-
high-density lipoprotein cholesterol ratio in Taiwanese
adults. Cardiovascular Diabetology, 10(93).
Er L. K., Wu S., Chou H. H., Hsu L. A., Teng M. S., Sun
Y. C., Ko Y. L., 2016. Triglyceride Glucose-Body
Mass Index Is a Simple and Clinically Useful
Surrogate Marker for Insulin Resistance in
Nondiabetic Individuals. PLoS ONE, 11(3).
Griffin S. J., Little P. S., Hales C. N., Kinmonth A. L.,
Wareham N.J., 2000. Diabetes risk score: towards
earlier detection of type 2 diabetes in general practice.
Diabetes/Metabolism Research and Reviews, 16(3):
164-171.
Keating B, J., 2015. Advances in Risk Prediction of Type
2 Diabetes: Integrating Genetic Scores with
Framingham Risk Models. Diabetes, 64(5):1495-1497.
Kuhn M., 2008. Building predictive models in R using the
caret package. Journal of Statistical Software, 28(5):
1-26.
Rutter M. K., Wilson P. W. F, Sullivan L. M., Fox C. S.,
D’Agostino R. B., Meigs J. B., 2008. Use of
Alternative Thresholds Defining Insulin Resistance to
Predict Incident Type 2 Diabetes Mellitus and
Cardiovascular Disease. Circulation, 117(8):1003-
1009.
Sweets J. A., 1988. Measuring the accuracy of diagnostic
systems. Science, 240:1285–1293.
Youden W. J., 1950. Index for Rating Diagnostic Tests.
Cancer, 32-35.
Models for Predicting the Development of Insulin Resistance
125
