
Architecture for a Combined Mobile Robot and Human Operator
Transportation Solution for the Hierarchical Life Science Automation
S. Neubert
1
, T. Roddelkopf
2
, X. Gu
2
, B. Göde
1
, S. Junginger
1
, N. Stoll
1
and K. Thurow
2
1
Institute of Automation, University of Rostock, Friedrich-Barnewitz-Str. 8, 18119 Rostock, Germany
2
Center for Life Science Automation (celisca) Friedrich-Barnewitz-Str. 8, 18119 Rostock, Germany
Keywords: Life Science Automation, Laboratory Execution System, Mobile Robot Integration, Dynamical Scheduling,
Human Machine Interaction.
Abstract: Modern laboratories for life sciences often include several different integrated automation systems to
increase throughput and quality, to reduce efforts for human operators and to reduce the costs of processes.
Typically, the planning and monitoring of methods are prepared and executed directly on local computers of
the automation systems. Moreover, a manual replenishing of resources and a manual transfer of samples and
labware between interacting automation systems are required in order to ensure end-to-end operations in a
24/7 mode.
This work describes the architecture and the pilot solution of a hierarchical workflow management system
(HWMS), which integrates distributed automation systems by combined use of mobile robots and human
operators as transportation units. With a graphical process design tool a material flow-oriented diagram can
be created, which describes the correlations of distributed subsystems in a complex workflow. The HWMS
schedules the workflows and controls the execution autonomously dependent on the planned process
diagrams. Two front-end components located on the process control layer simplify the integration and
support the control of the required subsystems. With smart device communications human operators can be
integrated in the workflow for transportation and assistance tasks as a necessary alternative to the robots.
1 INTRODUCTION
The 24/7 operation and the integration of automated
workstations are basic requirements in life science
processes to increase the throughput and the
processing quality and to reduce the effort of
monotonous processing steps and the risk of
potentially hazardous setups for laboratory assistants
(Patel et al., 2014; Lam et al. 2012).
A further reason to invest into automation in life
science laboratories is cost reduction (Cork and
Sugawara, 2002). However, currently only single
devices, automated workstations (e.g. liquid
handlers including peripheral devices) and integrated
automation systems (workstations, which include
one or more local transportation robots) are
commonly found in life science automation, as for
example seen in (Andersen et al., 2012; Liu et al.,
2010; Sutherland et al., 2014). Especially larger
systems consisting of distributed automated devices,
workstations and integrated automation systems –
here called automation systems – often provide
unresolved challenges regarding their complete
automation. The obvious reason is the non-existent
sample transportation control between the
automation systems and a required higher level
control system (complex automation system) for the
synchronization of all sub processes.
For preparation matters such as manual supply of
resources or a manual transport, especially in
systems with frequently changing tasks, the
assistance of a human operator is still essential. A
continuous progress of several running life-science
processes requires an appropriate involvement of the
operators. The operators must always be informed
about all necessary manual steps required to keep
the process chain running.
This article describes a development to increase
the level of automation in life sciences by setting up
a hierarchical control solution combined with mobile
robots and mobile devices in the instrument layer. In
modern laboratory automation systems, these
components enable higher system integration and a
higher degree of effectiveness by reducing the
Neubert, S., Roddelkopf, T., Gu, X., Göde, B., Junginger, S., Stoll, N. and Thurow, K.
Architecture for a Combined Mobile Robot and Human Operator Transportation Solution for the Hierarchical Life Science Automation.
DOI: 10.5220/0006423600410049
In Proceedings of the 14th International Conference on Informatics in Control, Automation and Robotics (ICINCO 2017) - Volume 2, pages 41-49
ISBN: Not Available
Copyright © 2017 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
41

waiting time between distributed sub processes. An
additional effect is the transition from automation as
part of a manual chain to manual steps as an integral
part of full automation in life science processes. This
transition is necessary due to the fact that non-
continuous interaction between human and machine
processes builds a bottleneck in the workflow,
especially when processes are executed parallel by
robots and involved humans. Thus, the
interconnection of robot- and human-controlled sub
processes needs to be managed for a continuous
effective workflow.
Personal digital equipment, such as smartphones
and tablet PCs, are often used in laboratories to
support the human operators. They allow to access,
e.g. laboratory information management systems
(LIMS) or electronic laboratory notebooks (ELN)
(Göde et al., 2007) or to use the wide range of
mobile device applications to calculate, to monitor
or to analyze data (de Souza, 2010). Mobile device
interfaces can also be found in special devices such
as the Optima XPN centrifuge of Beckman Coulter,
which can be monitored and controlled via an iOS
app (Muenz, 2012). Concerning more complex
applications smartphones and tablet PCs are a
comparatively new trend in life science laboratories.
2 ARCHITECTURE
The architecture of the developed systems follows
the typical automation structure of laboratory
automation as shown in fig. 1. The highest level in
this architecture is the hierarchical workflow
management system (HWMS). It is located in the
workflow control layer of the structured automation.
The name HWMS results from its position and
dependency to the complex hierarchical structured
sub-architecture below and it is classified as a
specialized manufacturing execution system (MES),
a so called laboratory execution system (LES). In
section III the HWMS is described in detail.
In the process control layer a general
differentiation is made between transportation and
automation systems' tasks, which is correspondingly
based on the two subsystems at the front end as
follows:
• The transportation and assistance control
system (TACS) permits the adaption and
distribution of transportation and assistance
orders from the HWMS and ensures their routing
to the transportation resources (pool of mobile
robots or alternative human operators).
Figure. 1: Architecture of the total system in the hierarchical structured laboratory automation.
ICINCO 2017 - 14th International Conference on Informatics in Control, Automation and Robotics
42

• The process control adapter system (PCAS)
consists of several instances, which interact
individually between the HWMS and the more or
less integrated automation systems, controlled by
process control systems (PCS) / scheduling
systems or vendor specific instrument control
systems (ICS).
Both systems adapt, execute and control the
required sub processes dependent on the orders of
the HWMS and return process-status information.
The systems are integrated by web-services-oriented
interfaces, which allow decoupled accesses from the
superordinated control systems. To relieve the
HWMS those systems have a sufficient application-
oriented intelligence to make own decisions for the
handling of sub processes
Dependent on the specificity of the order, the
TACS is able to dynamically schedule the
transportation and assistance orders for a planned
process between the available transport resources.
An internal list containing performance information
of all different transport resources allows an
automated selection of the best fitting mobile robot
or human operator. Each transportation resource
only receives one order at the same time, whilst the
orders can be more complex. For example, this can
be a labware transportation order of 20 different
objects (identified by barcode) or a list of assistance
orders to prepare and / or to monitor an automation
system (especially for human operators).
The group of transport resources is differentiated
into the following two options:
• The robot board center (RBC) executes the
transportation of labware via mobile robots (H20
Robot – Dr Robot Inc.). This unit includes the
navigation, the pick and place process, door
control procedures and the active collision
avoidance. The RBC requires the superordinate
robot remote center (RRC) in the process control
layer as central control instance for all mobile
robots. It has the responsibility for path planning,
central status information collection and decision
making regarding the most qualified robot,
which is selected by available power status and
the optimal current start position (Liu et al., 2012
& 2012 & 2013).
• The mobile human operator service (MHOS)
partly functions as a manual backup to the RBC.
However, by integration of human operators,
such as laboratory assistants, MHOS is
additionally able to provide orders for manual or
special tasks that cannot be performed by
automated systems. The MHOS is based on
mobile devices such as smartphones and tablet
PCs, which communicate directly with the
TACS.
The PCAS allows the connection to the involved
PCS to initiate runs of the automation systems for
complex laboratory tasks, including sample
preparation, dosage, analytical measurements, or
evaluation processes. General used PCS are complex
scheduling software systems such as SAMI® EX,
VWorks®, Microlab® VENUS or Clarity (Delaney
et al., 2013). Parallel to the automation systems with
PCS properties, single devices are required, which
can be integrated via the specified manufacturer
software (ICS) located in the instruments layer.
Therefore, the PCAS allows the direct access to the
instrument layer of the structured laboratory
automation and enables a higher integration
flexibility for the resource variety of the workflow
control layer.
A labware location guidance service (LLGS),
triggered by the PCAS, organizes the control of
special indicator systems to simplify human-
machine interactions. This refers for example to
complex labware storage hotels (400 positions) with
dynamic visual information that indicates the status
of every position slot to the human operator in
current and future processes of an automation line.
3 HIERARCHICAL WORKFLOW
MANAGEMENT SYSTEM
LES, like the HWMS, encapsulate complex
automation processes and ensure a stronger
decoupling of potentially superordinated control
systems (as e.g. the Business Process Management
System - BPMS) and the required automation
environment. Therefore, the HWMS includes the
complete adaption of the heterogeneous automation
systems' environments and simplifies the integration
of more real-time sensitive sub processes for the
end-to-end process automation (Neubert et al.,
2016).
The developed HWMS includes a relational
recursive database, which manages workflow
process definitions, workflow instances, locations,
labware, automation systems, mobile devices and
mobile robots. A web-portal allows the users and
system administrators to manage these environment-
tal parameters, which is most frequently required for
the definition of new labware and for printing the
appropriate barcodes. Based on these parameters an
Architecture for a Combined Mobile Robot and Human Operator Transportation Solution for the Hierarchical Life Science Automation
43

Figure 2: Material flow-oriented process model.
integrated graphical process design tool allows the
definition of an abstracted material flow diagram to
chain distributed heterogeneous PCS and ICS by
embedding all intersystem transport tasks in
laboratories (see fig. 2). Therefore, the user defines
the entry point for the labware into the workflow,
combines the required subsystems and determines
the already prepared methods of the PCS and ICS in
the process design tool. In practice these methods
are normally created for general procedures; thus it
can be used for different applications and
combinations. During the definition of the process
diagram the HWMS supports interactions with the
subsystems to receive planning-relevant information
(e.g. scheduling results of the PCS, labware start
positions of sub processes, status information) and to
implement them in the background into the technical
and the material-flow-oriented process model as e.g.
the number of the required labware path inputs and
outputs for a subsystems method execution
(see fig. 2). These labware paths allow to follow the
involved labware groups, which are defined by their
function in the process. In contrast to the labware
path input positions, which are defined in the start
conditions of a PCS-method, the output positions are
not always available during the planning phase. The
reasons for that are process-dependent logical
decisions in the PCS-method, which influence the
distribution of the labware on the final transfer
position (labware path output position). The HWMS
requests these output positions from the PCS during
the runtime, directly after finishing the subprocess,
to receive the actual positions of all paths.
The transportation tasks in the process model
will be integrated implicitly by the graphical linking
of the subsystems labware path inputs and outputs.
Process-model options allow e.g. the definition of
ICINCO 2017 - 14th International Conference on Informatics in Control, Automation and Robotics
44
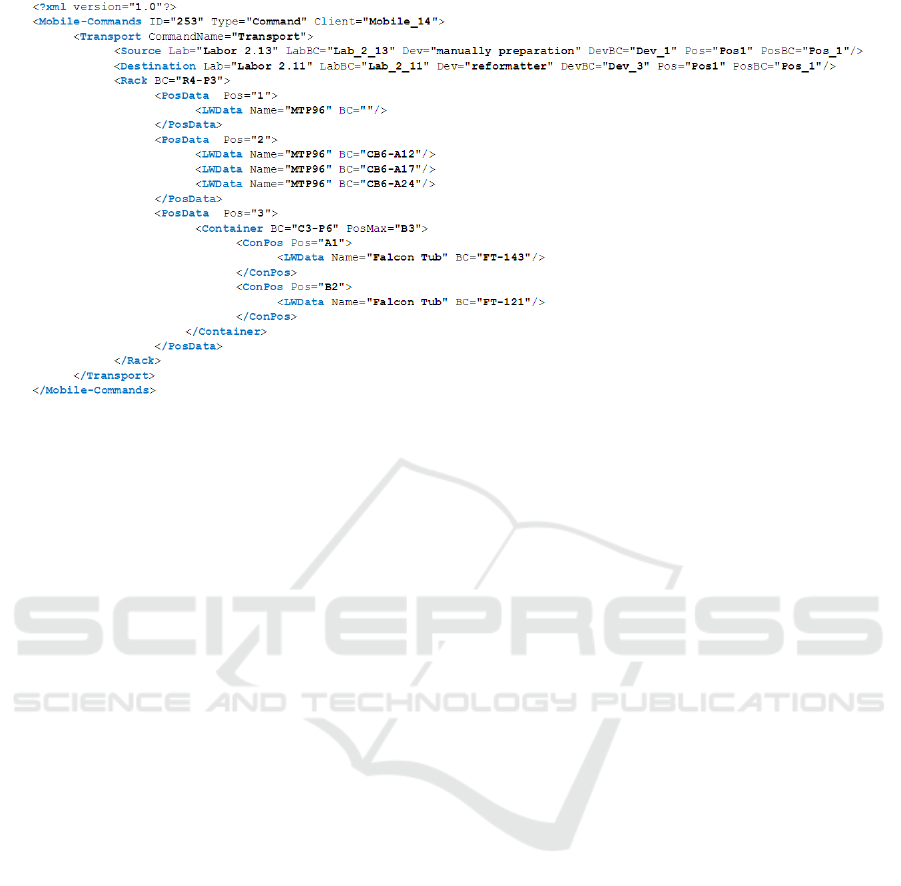
Figure 3: Comunication protocol on the basis of xml.
the kind of transporter (mobile robot or human
operator). In the technical process model the
executability of the transporter decision will be
validated and corrected if necessary. Alternatively
this decision can also be passed to the TACS, if both
kinds of transporters can execute the specific task.
The end point determines the final position for the
labware after the competition of the workflow. The
complexity and the required usability of the process
diagram demand a specified notation as applied,
which can be very complex when using standards
from higher level control systems as e.g. BPMN 2.0
(Business Process Model and Notification) (OMG,
2011).
For the execution of the planned process
workflow, the material flow diagram has to be
translated in a processible format, separated in
logical sub processes (primary divided into transport
and automation system processes) and scheduled
together with all workflows to be performed. For the
latter a scheduler in the process controller is used,
which works with a genetic algorithm. It generates
an optimized execution order of the sub processes in
consideration of the limited numbers of resources
(e.g. applicable integrated automation systems or
transport units) and assigned priorities (Gu et al.,
2016).
Depending on the scheduling results the process
controller verifies the subsystems and triggers the
required subprocesses to execute arranged methods
or tasks (e.g. labware transport, pipetting process by
a liquid handler) on them, after starting the
execution by the user. The execution will be
assumed by the structured subsystems on the
subordinated process-control and instrument-control
layer.
Due to the fact that the conditions of the
environment can change, for example by newly
started workflows, which have to be implemented in
the total workflow, by changings in the number of
available transportation units (robots have to charge
or can be broken, human operators have a limited
working time or have to fulfil other tasks) or by
unforeseeable delays, which can occur especially
during the transportation processes, a dynamic
scheduling is required (Schäfer, 2004). It is realized
by a rescheduling of the total workflow during the
execution and is triggered by the detection of such
changings (delay measurements or notifications
about the available number of transportation units).
4 INTERACTION AND
COMMUNICATION
The HWMS is a web-based telematics platform,
which is primarily integrated by the laboratory's
computing environment. It provides the interfaces to
structured automation systems and considers the
usability of process definition and execution for a
wide range of life science laboratories. Thus, the
data transfer between the HWMS and the process
control layer's front-end systems is based on web-
services, which allow a simple and flexible
integration of all web-service providing subsystems.
The HWMS uses this to send process instructions
and to request the status of the front-end subsystems
to correlate the information and to initiate required
Architecture for a Combined Mobile Robot and Human Operator Transportation Solution for the Hierarchical Life Science Automation
45

Figure 4: UML Sequence diagram of the communication process between HWMS and the transportation control system.
processes in the subordinated architecture, following
the scheduling result.
In case of the PCAS, every PCS or ICS has a
preceded individual service instance, which uses
available service-oriented communication or
framework interfaces to access the automation
systems for workflow control. During the execution
of a sub process, the PCAS observes the running
process and offers status and error information on
request to the HWMS to inform the requesting user
and to resume the process chain.
Main function of the TACS is the distribution of
transportation orders for robots and human
operators. The TACS works centralized and
provides, in analogy to the PCAS, a web-service for
the communication to the HWMS. Depending on the
availability of the operators, the restrictions of the
user for the process and the reliability of the
different operators for the current order, the TACS
decides whether a robot or the human operator is
more qualified or can finish the order faster.
Furthermore, the TACS distributes assistance orders
such as sample preparation, restocking resources at a
workstation, prepare a laboratory automation system
for other processes or check their status after
execution faults. Currently these kinds of orders can
only be done by human operators.
The communication between the TACS and the
transportation resources (RRC or MHOS) is realized
by using a flexible XML-based message protocol
transmitted via TCP or UDP, dependent on the unit's
type. For the mobile robots, the RRC represents the
remote station to the TACS, which uses TCP-
communication and forwards the orders to the
requested robot (Liu et al., 2012). On the other hand,
the TACS integrates the separated MHOS units
based on several mobile devices via the UDP
protocol. The properties of UDP allow the TACS to
communicate with an almost unlimited number of
MHOSs without a continuous connection to the
single devices. It is less dependent on possible
connection interruptions, which may occur when
human operators leave the local wireless network.
Both kinds of transport resources work with a
highly structured XML-message protocol, which
contains all relevant information, such as the places
of source and destination and the list of labware
including the barcode. In fig. 3 a transportation order
is shown, which is based on a general transportation
rack with three positions in the ANSI/SBS footprint
ICINCO 2017 - 14th International Conference on Informatics in Control, Automation and Robotics
46

dimensions for microtiter plates. On these positions
combinations of microtiter plates and adapting racks
for different kinds of tubes, also in stack format, are
possible.
For every message dispatched by the TACS a
confirmation concerning the correctly received
command is expected. This implies completeness,
correct decompression and readability of the
message. After having finished the order, the
executive transport resources will send a confirming
reply back to the TACS. In fig. 4 the communication
cycle between HWMS, TACS and both kinds of
transportation resources are visualized in a sequence
diagram.
5 MOBILE HUMAN OPERATOR
SERVICE
As an alternative to the transportation by the mobile
robots, which is described more precisely in (Liu et
al., 2012 & 2012 & 2013), human operators are still
required for particular processing steps or for
limitations of robot pools and for special
transportation orders in life science processes. To
include a group of human operators into the
automated process, every single operator needs a
separate mobile interface to be flexible and to
receive orders from the TACS. Therefore, Android-
based mobile devices are used, which are available
in different formats. In general, mobile devices
combine a flexible I/O-interface with high
connectivity and other functionalities, such as
integrated cameras.
Via an Android-based laboratory application
(LApp), the human operators are logging-in
themselves to the TACS. Thus, they are registered as
being available to receive transportation and
assistance orders, dependent of the operator’s
individual role. All orders are listed and can be
selected by the operator to see more details and
further handling. A graphical overview of the
transportation order allows seeing the expected
positioning of the labware for the process on the
destination side. In case of preparing an automation
system, this has a determined arrangement of the
labware dependent on the current method. These
arrangements can consist of up to three stacks, each
with up to ten single microtiter plates or other
labware (see Fig. 5). The arrangement needs to be
maintained to avoid mistakes and to prevent the
workstation from re-sort processes, especially if the
space is limited.
Figure 5: Screenshot of the mobile device list and
graphical user interface with the required transport
arrangement of the labware.
To reduce the effort for the human operator, the
mobile device camera is used to identify the
labware's barcode and to allocate a labware to a
position in the arrangement, when the position is
allowed to be edited. This can appear when a
workstation is equipped with empty labware of the
same type. In this case, the MHOS initializes the
start position for the sample tracking across the
overall process.
Furthermore, the HWMS and even the integrated
stock management are available via the mobile
device's web browser. In this case, the human
operator can register, re-sort or find goods in the
stock management, which can be distributed all over
the institution. Label printers also enable the printing
of self-defined barcodes to identify new labware. All
these functionalities are also obtainable via
stationary computers.
6 CONCLUSION
This paper describes an architecture to combine
distributed conventional hybrid and heterogeneous
automation systems with mobile robots and human
operators (integrated by mobile devices) to solve the
demands of automated transportations between
Architecture for a Combined Mobile Robot and Human Operator Transportation Solution for the Hierarchical Life Science Automation
47

them. Thus, the automation level in life science
laboratories can be increased and the human
operators can also be integrated in required
assistance tasks in runtime.
The HWMS allows the handling of master data
and stock management, which is the basis for
planning life science processes over several
automation systems. By an integrated process design
tool the end user can plan workflows including
theses automation systems by a material-flow-
oriented process model. The resulting models can be
executed parallel by means of a dynamic scheduler
(based on genetic algorithm) to optimize the use of
the required resources (e.g. automation systems,
mobile robots, human operators) in the complex
workflow. Variable transfer positions between the
automation systems are also tolerated by the
HWMS. By using mobile robots, HWMS
accomplishes an important requirement of life
science automation as a complex process connector
between distributed automation systems in a
building.
The TACS and the PCAS build the front end of
the process-control layer and share the control over
the automation systems as well as the mobile robots
and mobile devices. Both systems distribute and
adapt the orders for the corresponding automation
subsystems, whereby the TACS is able to conduct
dynamic transportation-unit allocations for each
intersystem transport process. For the
communication between the TACS and the
subsystems, a uniform XML-message-protocol via
TCP and UDP is used. The PCAS consists of
individual services on the local computers of the
heterogeneous automation systems to implement the
whole performance range of the systems.
Especially the MHOS unit, available on smart
phone or tablet PC, integrates laboratory assistants
increasingly in the running workflow. The
requirements of the system, which cannot be
performed by mobile robots, will be directly
submitted via transportation or assistance orders to
human operators.
In summary, the presented workflow
management system located on the workflow control
layer is able to speed up the laboratory work in
general, to reduce the effort for human operators and
to combine human and machine in an automated or
semi-automated process albeit this puts the
observation function for the human operators more
in the center stage. Thus, the automated system
receives the control role concerning time
management, while the focus of the human operators
is on manual preparation steps and on observing the
process as a whole.
Although humans currently transport faster than
the robots, used in this solution, the process-
integrated robot operators have the advantage of
being able to react immediately to a command, to
manage dangerous transportation orders and to
operate 24/7. Thus, although the advantages of
human und robot operators differ, they are both
useful depending on the respective requirements and
a parallel availability of both operators cover
processes around-the-clock and high priority or
special transportation orders, too.
ACKNOWLEDGEMENTS
The authors thank the Ministry for Economic
Affairs, Construction and Tourism of Mecklenburg-
West Pomerania
(Germany, FKZ: V-630-S-105-
2010/352, V-630-F-105-2010/353
) and the Federal
Ministry of Education and Research (FKZ:
03Z1KN11, 03A1KI1) for the financial support of
this project. This work has been supported by the
European Union.
REFERENCES
Patel, S. N. Prajapati, K. R. Sen, D. J., 2014. Automation
by Laboratory Robotics in Pharmaceutical Research
Industry: A latest venture in innovative idea. In: World
Journal of Pharmacy and Pharmaceutical Science
(WJPPS), vol.3 (2), pp. 2098–2105.
Lam, C. W., Jacob, E., 2012. Implementing a laboratory
automation system: experience of a large clinical
laboratory. In: J Lab Autom, vol.17 (1), pp. 16–23.
Cork, D. G., Sugawara, T., 2002. Laboratory automation
in the chemical industry. New York: Marcel Dekker.
Andersen, D., Rasmussen, B., Linnet, K., 2012. Validation
of a fully automated robotic setup for preparation of
whole blood samples for LC-MS toxicology analysis.
In: J Anal Toxicol, vol. 36 (4), pp. 280–287.
Liu, L., Zhang, R., Pajak, L., 2010. Automation of Human
IgG, Glucose, Lactate, and Oxygen Assays on Biomek
NXP Span-8 Automation Workstation and
PARADIGM Detection Platform. In: Journal of the
Association for Laboratory Automation, vol. 15 (5),
pp. 414–418.
Sutherland, J. D., Tu, N. P., Nemcek, T. A., Searle, P. A.,
Hochlowski, J. E., Djuric, S. W., Pan, J. Y., 2014. An
Automated Synthesis-Purification-Sample-Manage
ment Platform for the Accelerated Generation of
Pharmaceutical Candidates. In: Journal of Laboratory
Automation, vol. 19 (2), pp. 176–182.
ICINCO 2017 - 14th International Conference on Informatics in Control, Automation and Robotics
48

Göde, B., Holzmüller-Laue, S., Rimane, K., Chow, M.-Y.,
Stoll, N., 2007. Laboratory Information Management
Systems – An Approach as an Integration Platform
within Flexible Laboratory Automation for
Application in Life Sciences. In: Proc. of the 3rd
Annual IEEE CASE, Scottsdale, AZ, USA, Sept 22-25,
pp. 841–845.
de Souza, N. (ed), 2010. The scientist and the smartphone.
In: NatMet, vol. 7 (2), p. 87.
Muenz, R., 2012. The right choice of centrifuges. In: Lab
Manager, p. 51.
Liu, H., Stoll, N., Junginger, S., Thurow, K., 2012. A
common wireless remote control system for mobile
robots in laboratory. In: Proc. of the IEEE I2MTC
Conference 2012, pp. 688-693.
Liu, H., Stoll, N., Junginger, S., Thurow, K., 2013. An
Application of Charging Management for Mobile
Robot Transportation in Laboratory Environments. In:
Proc. of the IEEE I2MTC Conference 2013, pp. 435-
439.
Liu, H., Stoll, N., Junginger, S., Thurow, K., 2012. A
Floyd-Dijkstra Hybrid Application for Mobile Robot
Path Planning in Life Science Automation. In: Proc. of
the 8
th
IEEE International CASE 2012, Seoul, Korea,
pp. 279-284.
Delaney, N. F., Echenique, J. I. R., Marx, C. J., 2013.
Clarity: an open-source manager for laboratory
automation. In: J Lab Autom, vol.18 (2), pp. 171–177.
Neubert, S., Göde, B., Gu, X., Stoll, N., Thurow, K., 2016.
Potential of Laboratory Execution Systems (LESs) to
Simplify the Application of Business Process
Management Systems (BPMSs) in Laboratory
Automation. In: JALA, Online First, December 2016.
OMG. Business Process Model and Notation (BPMN)
Version 2.0. 2011. http://www.omg.org/spec/BPMN/
2.0
Gu, X., Neubert, S., Stoll, N., Thurow, K., 2016 Intelligent
Scheduling Method for Life Science Automation
Systems. In: Proc. IEEE International Conference on
Multisensor Fusion and Integration 2016, pp. 156-
161.
Schäfer, R., 2004. Concepts for dynamic scheduling in the
laboratory. In: JALA, vol.9 (6), pp. 382–397.
Architecture for a Combined Mobile Robot and Human Operator Transportation Solution for the Hierarchical Life Science Automation
49
