A Model Oriented Approach for Managing Traceability of Biological
Samples and Tests of Patients in Assisted Reproduction Clinics
L. Morales-Trujillo
1
, V. Cid de la Paz Furest
1
, J. G. Enríquez
1
and José Navarro
2
1
Computer Languages and Systems Department, University of Seville, Avenida Reina Mercedes, s/n 41010, Sevilla, Spain
2
Assisted Reproduction Clinic - Inebir, Hospital Victoria Eugenia, Avenida la Cruz Roja, 1, 41009, Sevilla, Spain
Keywords: Traceability, Control Process, Assisted Reproduction.
Abstract: Assisted reproduction has become a service that more and more people access. Current problems such as the
delay in the age of motherhood, single-parent couples, etc. they have proliferated the options and the different
treatments that are put at the service of society. A fundamental part of these processes lies in the work of
laboratories, in the samples that are handled in clinical processes and then be implanted in future mothers.
The management of the samples is a critical aspect that requires all the opportune mechanisms that guarantee
the traceability of said samples, avoiding fatal errors. The correct identification, monitoring and control of
them is a fundamental aspect and of special relevance. However, the systems currently offered to clinics
present important problems. On the one hand, they offer little security, are very expensive or very independent
of a specific provider, so that the traceability system cannot be connected to the hospital central management
system, or they are very intrusive control systems in the daily work of the laboratory technicians. In this paper
a software solution based on the definition of automatic models and protocols is proposed. It includes the
appropriate devices, to manage the traceability of the samples in parallel to the work of the laboratory
technician.
1 INTRODUCTION
For some years now, assisted reproduction techniques
have been part of the reproductive history of many
couples. According to the most extensive
epidemiological studies, infertility affects 15% of the
population of reproductive age in Western countries,
that is, one in six couples, and experiences an
increasing evolution (País, 2017).
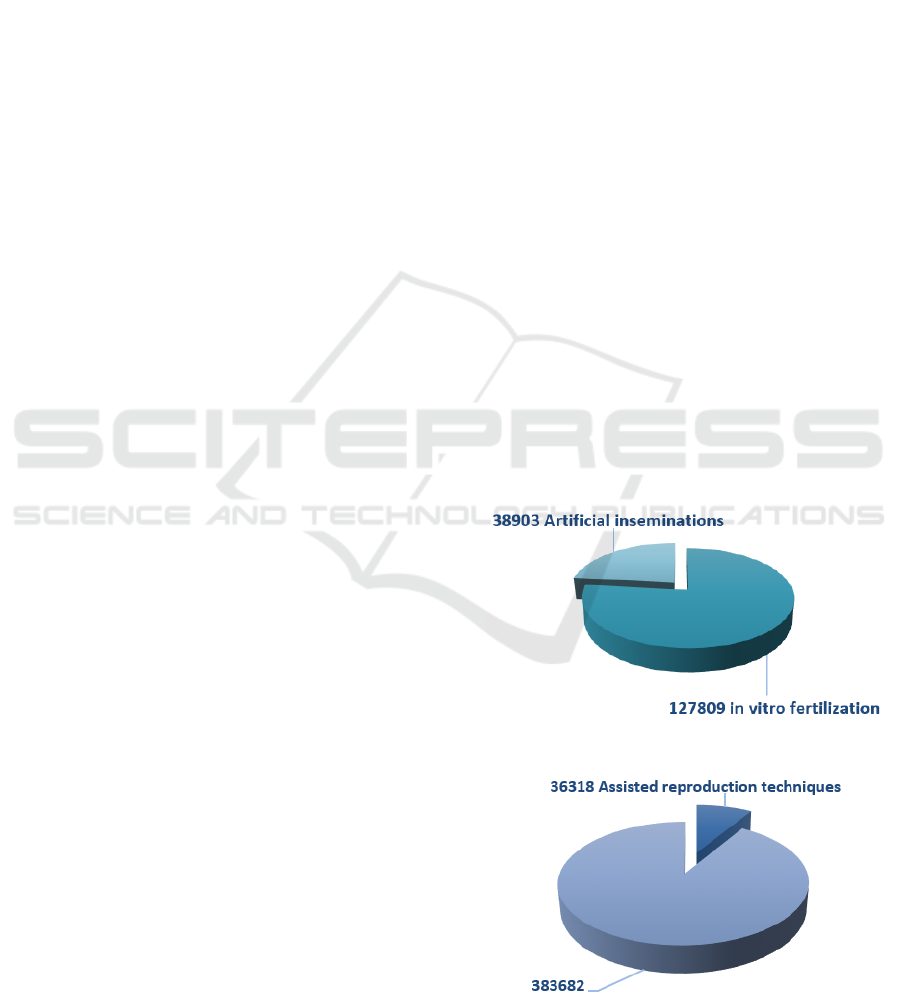
Spain is a leader in Europe in assisted
reproduction techniques, with a total of 127,809 'in
vitro' fertilization cycles and 38,903 artificial
inseminations in 2015. In 2015, a total of 36,318
children were born in Spain thanks to assisted
reproduction techniques, representing 8,6% of the
more than 420,000 births that occurred that year,
according to the latest data from the Ministry of
Health (Mundo, 2017). Figure 1 and 2 show some
results graphically.
Figure 1: Assisted reproduction techniques, Spain 2015.
Figure 2: Children born in Spain in 2015.
Morales-Trujillo, L., Furest, V., Enríquez, J. and Navarro, J.
A Model Oriented Approach for Managing Traceability of Biological Samples and Tests of Patients in Assisted Reproduction Clinics.
DOI: 10.5220/0006961003010307
In Proceedings of the 14th International Conference on Web Information Systems and Technologies (WEBIST 2018), pages 301-307
ISBN: 978-989-758-324-7
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
301

However, although this has allowed the centres to
reach technological and service levels "leading", has
also put Spain "in the crosshairs" of many European
scientific societies and groups of other countries,
since we still have a lot for improving in terms of
control mechanisms (BMD, 2016).
Currently there is no control and transparent
system for the professional to verify if the biological
samples and patient tests are right, which prevents
serious errors. This paper marks the challenge of
increasing trust on the part of the patients involved by
ensuring the control and monitoring of the samples
and tests of the patient in such a way that the risk of
error is reduced to minimum levels by proposing a
software solution based on the definition of automatic
models and protocols, which by incorporating the
appropriate devices, control the traceability of the
samples in parallel to the work of the laboratory
technician. This solution must be affordable and
compatible with the standards that govern hospital
management processes and laboratory samples in
assisted reproduction.
2 PROBLEM
Human reproduction laboratories are exposed to
multiple incidents, but one of the most serious is that
which causes the erroneous identification of
biological samples (ovules, sperm and embryos),
being the misidentification a common problem in all
areas of health.
An identification error occurs when a patient is
incorrectly paired with a test, treatment or procedure
and is usually caused by stress, work overload,
multiple interruptions, material errors, among many
other factors.
University Hospital Utrecht has made public the
possible fertilization of oocytes of 26 women with
sperm outside their partner, that is, a score of women
or couples have sired children with the sperm of the
man who was not the indicated. Finally, the center
detected that the pipette that had been used in some
oocyte fertilization procedures was contaminated
with the sperm of another patient (País, 2016).
Certain processes, such as the mixture of ovules
and sperm, and the transfer of embryos to the uterus,
are seen as critical, since they represent "the point of
no return." If an erroneous identification is produced
in assisted reproduction laboratories, it can be go
unnoticed practically in each of the steps of the
process involving gametes and embryos. The final
result will be catastrophic for both the patient, as for
the professional and the clinic, with legal implications
that can lead to sanctions and even, in extreme cases,
at the close of the clinic.
One of the most used solutions to avoid these
possible errors, is the manual double-test protocol
(MDT), defined as the obligation of "double control
performed in all clinical and laboratory procedures"
with the expectation that, if a " operator "makes a
mistake, it will be captured by the other" witness "in
time to be resolved. However, the evidence suggests
that it may not be as safe and effective. The effects of
mechanization of tasks can reduce the effectiveness
of double testimony because the levels of attention
decrease when the same action is carried out
repeatedly by the same person. Therefore, the risk of
error during the double-checking protocol may
increase due to numerous problems, such as process
redundancy, attentional blindness, ambiguous
responsibility, errors in the verification, additional
work overload and increased stress. In addition, the
process of double control produces additional
"paperwork" to an already overwhelming work
environment and entails the duplication of resources
in an already expensive process.
For these reasons, several alternatives have been
developed based on less manual identification
techniques in order to replace most of the steps
required by human witnesses in assisted reproduction
laboratories. The most widespread are:
Systems based on barcode labels, which,
moreover, are often used in collaboration with
MDT protocols. It consists of identifying the
pipettes with a bar code so that as a process is
executed in the laboratory, the technician uses
a reader of said bar code to collect the life cycle
of the sample. In this regard, it is expected that
the "Single European Code" (SEC) will be
launched soon, which would increase
confidence in the realization of these
techniques in a quality framework (RHA
Professional). However, this code consists of
40 characters, too large to be placed in
cryopreservation devices: Semen, oocytes and
embryos are conserved in devices called straws
or vitrification supports. Even a simplification
is not feasible through the use of 20 characters
(RHA Professional).
Systems based on silicon bar codes that are
injected directly into the ovules or embryos,
work in a similar way but the identification is
found in the sample itself. This option is a bit
aggressive in the eyes of the donor of the
sample, since a marker is being introduced to
his biological sample, to his possible son.
WEBIST 2018 - 14th International Conference on Web Information Systems and Technologies
302

Systems based on radio frequency
identification (RFID) technology RFID
systems involve placing an adhesive RFID tag
on all fungibles that contain embryos and
gametes related to a single patient. These labels
are analysed in each station of the process to
identify the patient with whom you are working
at that moment. A digital record keeps track of
the location of each label at each stage of the
overall process and which staff member is
manipulating the samples. Visual and auditory
alarms indicate when an imbalance has
occurred so that it can be corrected promptly.
The RFID system also keeps a record of the
imbalances, so it is possible to review and
analyse the different stages, detecting the steps
with the highest risk in order to reduce the
imbalances accurately. There are already
companies that have developed solutions, with
a high cost, based on RFID, such as Cooper
Surgical Company and its RI Witness.
However, these solutions have little
adaptability to different laboratory concepts,
are not affordable and are closed to the specific
fungibles of the companies, making it difficult
to intercommunicate with the rest of the
hospital management system.
In short, there is currently no control system,
transparent for the professional, to verify if the
biological samples that are being worked on are those
indicated, which prevents serious errors from
occurring.
For all this, our research aims to create a
technological solution that allows to take control of
the work of technicians in assisted reproduction
laboratories when they execute processes that involve
work with samples. This solution should be little or
nothing invasive in the work of the technician,
compatible with the management system and
affordable and adaptable to the different realities of
assisted reproduction clinics. It must also guarantee
that any sample identification must be in accordance
with current regulations and follow the guidelines set
by the Ministry of Health.
3 GOALS
This project marks the challenge of designing a
strategy that reduces the risk of error minimum levels
making use of information technologies as the central
axis of the solution. The objectives and requirements
defined to achieve it are described below.
The main objective of the research is the
definition and implementation of an ICT solution,
which can be integrated in a simple way in the
processes that follow the biological samples to
provide a complete, univocal and safe traceability of
each sample during its life cycle.
In order to carry out this objective, we propose the
next research steps.
Study the current situation in the identification
and monitoring of samples in assisted
reproduction.
Define a protocol to incorporate the monitoring
of the life cycle of biological samples and tests
in a non-invasive and safe way in the work of
health personnel.
Develop a technological solution that allows
executing the protocol in an affordable and
adaptable way to the reality of the different
clinics.
Validate the solution in a real context.
4 HYPOTHESIS AND SOLUTION
PROPOSAL
The solution to be developed to achieve these
objectives must be composed of the following
aspects:
4.1 Theoretical Framework
For the management of traceability in clinics, which
provides alignment with traceability management
standards and allows the design, development and
application of a theoretical framework, which, among
other things, allows:
A work methodology for the management of
traceability biological samples and patient tests
based on standards, good practices and
international standards on biological samples
and patient tests that contribute to structure and
order the activities, documents or controls that
are planned and implemented inside a clinic.
The definition of the life cycle of the
traceability management process focused on
biological samples and patient tests that
includes planning, compliance, evaluation and
the correct adaptation of the standards that have
been contemplated in the proposal.
Stabilization of a standard does not only entail
A Model Oriented Approach for Managing Traceability of Biological Samples and Tests of Patients in Assisted Reproduction Clinics
303

compliance with it, it also implies the
mobilization of the clinic in a process that
ensures its future fulfilment.
Following the paradigm MDE (Model-Driven
Engineering) will be defined:
A meta-model of reference that describes
the processes and artefacts necessary to
carry out the entire life cycle of the
management of the traceability of
biological samples and tests of the
patient in the clinic. This metamodel will
serve as a basis to verify the control and
monitoring of all biological samples and
patient tests.
A series of checking mechanisms
between the reference metamodel and
the metamodel that is instantiated or
executed in the information system.
4.2 Why the MDE Paradigm?
Model-Driven engineering (MDE) is one of the most
deeply rooted paradigms in the area of software
engineering. It focuses on creating and exploiting
domain models, which are conceptual models of all
the issues related to a specific problem. Therefore,
highlight and point out abstract representations of
knowledge and activities that require a particular
application domain, instead of computer concepts.
An important aspect when using MDE is to
guarantee traceability between the generated process
models. This is essential in the context of the proposal
that is made here and that allows maintaining the
identity of a process among all the modules that
guarantee traceability and the possibility of finding
errors in the early stages, thus avoiding irrecoverable
failures.
By ensuring that the traceability between the
different processes that have to be carried out in a
technique of assisted reproduction and avoiding
possible irrecoverable failures during this process, the
levels of error are reduced to minimum levels and
they provide greater security to patients who want to
undergo a process of assisted reproduction.
After explaining why we use MDE in our
proposal, it is important to know what it is.
MDE came up in order to tackle the complexity of
platforms and the inability of third generation
languages to relief this complexity and effectively
express the domain concepts of the problem. This
new paradigm, apart from raising the level of
abstraction, intends to increase automation during the
life cycle of software development.
This paradigm works, as the primary form of
expression, with definitions of models and
transformation rules among these models which
entail the production of other models. Every model
corresponds to a phase of the life cycle and is
generally specified by means of UML modelling
language.
Standardization was necessary in order to
implement this new paradigm in real projects. OMG
presented MDA, which stands for Model-Driven
Architecture (OMG, 2003), as a platform to support
the paradigm of Model-Driven Engineering.
The main ideas of MDA consist in dividing the
specification of the system functionality from its
implementation on a specific technology platform, as
well as control the evolution from abstract models to
implementations. Thus, the degree of automation
usually increases. MDA proposes to base the software
development on models which make transformations
be performed to generate code or another model with
characteristics of a particular technology (or lowest
level of abstraction). As transformations go on, it may
be noticed that the models become more concrete and
the abstract model changes into another one
compatible with a particular technology or platform.
MDA is based on four types of levels or models:
The CIM level (Computation-Independent
Model) is considered the highest level of
business model and the most abstract level. It
focuses on requirements specification and
intends that anyone who knows the business
and its processes can understand a CIM model,
as this avoids any contact with the specific
system.
The PIM level (Platform-Independent
Model) represents the business process model
and system structure, without any reference to
the platform on which the application will be
implemented. It is usually the entry point for all
the support tools for MDA.
The PSM level (Platform-Specific Model)
specifically relates to the platform where the
system will be implemented, for example, with
operating systems, programming languages or
middleware platforms, among others.
Finally, the Code level refers to the codification
and suitable implementation of the system.
Figure 3 (Koch, 2006) represents a diagram with
the adaptation of the MDA standard in Web
development.
WEBIST 2018 - 14th International Conference on Web Information Systems and Technologies
304

Figure 3: Model-Driven Web Engineering.
In this context, a set of metamodels in the CIM
level is given which are requirements models. These
models allow information requirements to be
captured.
Analytical models are obtained systematically by
means of the transformation CIM-to-PIM: content
model, navigation model, presentation model, and
some others. In addition, PIM level allows the
application of some transformations (PIM-to-PIM) in
order to get design models. Subsequently, models on
the PSM level are obtained by applying
Transformations PIM-to-PSM. Finally, the
application of Transformations PSM-to-Code
generates the system code.
4.3 Support Tool
The proposal of the solution will be supported by a
support tool that allows accessibility from any
position or workplace to the management process of
biological samples and tests of the patient of the clinic
with secure access through user profiles. In addition,
it will act as a document manager of all the
information generated in the process. On the other
hand, a module that allows to represent the clinical
process will be required. This system must be
compatible with the central hospital management
system, be in accordance with the standards and allow
the administrator, or person who defines the process,
to "mark" the points that have to be controlled.
5 VALIDATION
A module that allows to represent clinical processes
and artefacts is required. This system must be
compatible with the central hospital management
system, be in accordance with the standards and allow
the administrator, or person who defines the process,
to even "mark" the most critical points that have to be
controlled.
On the other hand, a second module will be
developed that, by means of a specific device,
automatically, and based on the process itself, make a
control of those control points and refer them to the
central hospital management system, launching an
exception in the case the technician makes an error.
Finally, there will be a module to control and
monitor biological samples and patient tests that will
allow accessibility from any position or workplace to
them and, in addition, act as a document manager of
all the information generated in the process.
For its validation, a case study will be made taking
the Inebir clinic as a reference (Schmidt 2016). Inebir
is an assisted reproduction clinic located in Seville
(Spain), more specifically in the Victoria Eugenia
Hospital. This clinic has a laboratory designed and
built following strict guidelines that result in a work
space with the latest technological advances in the
field of fertilization, where a team of highly qualified
embryologists follows an exhaustive method of work
(García-García, 2017).
Next, 4 activity diagrams are presented where 4 of
the processes that are carried out in the laboratory of
the Inebir clinic are illustrated (Figure 4 to 7). These
Figure 4: Preparation and extraction of ovules.
A Model Oriented Approach for Managing Traceability of Biological Samples and Tests of Patients in Assisted Reproduction Clinics
305

diagrams can be visualized, apart from the sequence
of steps followed to carry out the corresponding
process, the actors that are involved in this process
and the places where they are carried out:
The first process that is illustrated is that which
goes from the extraction of oocytes from a patient, to
the conservation of the ovules extracted from said
oocytes.
Afterwards, you can visualize the process that
goes from the collection of semen samples to your
treatment so that an ovule can be fertilized later.
Figure 5: Preparation of semen samples.
The final step to which all the processes described
here are directed is the one that can be visualized in
this diagram, where the fertilization of the ovules and
the subsequent transfer to the uterus of a patient is
described.
Figure 6: Fertilization and transfer.
Finally, the last process shown here is optional. It
is the process that results in the freezing of samples.
Figure 7: Freezing of samples.
For all this, it is a perfect scenario to validate the
proposal presented here.
6 EXPECTED RESULT
The following are the results that await the solution
presented here in a process of assisted reproduction:
Greater control and monitoring of biological
samples and tests of patients reducing the risk
of error to minimum levels, an aspect that is
fundamental and of special relevance to
guarantee safety.
Increase the confidence of the patient since
reducing the risk of error brings greater peace
of mind.
WEBIST 2018 - 14th International Conference on Web Information Systems and Technologies
306

Improve the common understanding and
facilitate the continuous improvement of the
clinic in terms of the implementation of
standards, standards and good practices carried
out by third parties.
7 CONCLUSIONS
The paper presents a global view of a model-driven
approach to work with traceability in laboratories.
This approach was obtained for a real necessity in the
industry
The paper introduces the current situation and
analysis the problem that it exists in the field of
human reproduction.
We have, however a lot work to do. Fortunately,
we count with a real clinic support and one of our
main advantage is the real connection with users.
The use of the Model-driven paradigm also results
a suitable and promised idea. In fact, we have used in
other important areas with successful results (García-
García, 2012; Escalona, 2013).
ACKNOWLEDGEMENTS
This research has been supported by Pololas project
(TIN2016-76956-C3-2-R) of the Spanish Ministry of
Economy and Competitiveness.
REFERENCES
País, (periódico el País), «España lidera la reproducción
asistida en Europa,» 2017. [En línea]. Avalaible:
https://elpais.com/elpais/2017/07/04/mamas_papas/14
99166213_758427.html.
EL MUNDO, «El 8,6% de los niños nacen en España
gracias a técnicas de reproducción asistida,» 2017.
Available: http://www.elmundo.es/ciencia-ysalud/
salud/2017/10/11/59ddebb0ca474103188b45a1.html.
BMD (Banco Mundial de Datos) «Tasa de fertilidad, total
(nacimientos por cada mujer), Banco Mundial de
datos,» 2016. Avalaible: https://datos.banco
mundial.org/indicador/SP.DYN.TFRT.IN?view=chart.
EL PAÍS, «Holanda investiga la posible fecundación de 26
mujeres con esperma equivocado,» 2016. Avalaible:
https://elpais.com/internacional/2016/12/29/actualidad
/1483021366_741815.html.
RHA Profesional, «El Código Único Europeo. Toda la
información necesaria para entender su
funcionamiento,» Avalaible: http://www.rhapro
fesional.com/codigo-unico-europeo-ya-puedeconsulta
do-traves-la-plataforma-creada-la-comision-europea/.
Schmidt, D. C. Model-Driven Engineering. IEEE
Computer, Computer Society, vol. 39, no. 2, pp. 25-31,
2006.
Garcia-Garcia, J. A., Enriquez, J. G., Garcia-Borgonon, L.,
Arevalo, C., Morillo, E.: A MDE-based framework to
improve the process management: The EMPOWER
project. In: Proceedings - 2017 IEEE 15th International
Conference on Industrial Informatics, INDIN 2017
(2017).
García-García, J. A., Ortega, M. A., García-Borgoñon, L.,
& Escalona, M. J. (2012, July). NDT-Suite: a model-
based suite for the application of NDT. In International
Conference on Web Engineering (pp. 469-472).
Springer, Berlin, Heidelberg.
Escalona, M. J., García-García, J. A., Mas, F., Oliva, M., &
Del Valle, C. (2013). Applying Model-Driven
Paradigm: CALIPSOneo Experience. In CAiSE
Industrial Track (pp. 25-32).
OMG, 2003. MDA Guide of OMG. Version 1.0.1,
http://www.omg.org/docs/omg/03-06-01.pdf
Koch, N., Zhang, G., Escalona, M. J., 2006. Model
Transformations from Requirements to Web System
Design.
A Model Oriented Approach for Managing Traceability of Biological Samples and Tests of Patients in Assisted Reproduction Clinics
307
