
Influencing Factors and Kinetics of Degradation of Unsym-
Dimethylhydrazine Waste Water by H
2
O
2
/UV/O
3
Process
Zelong Xu
*
, Lingzhi Huang, Fei Chen and Yi Wu
China Jiuquan Satellite Launch Centre, Gansu Jiuquan 732750.
Email: 422412620@qq.com
Keywords
: Unsym-dimethylhydrazine (UDMH), ozone (O
3
), Ultraviolet (UV), hydrogen peroxide
Abstract:
The treatment of unsym-dimethylhydrazine (UDMH) waste water through H
2
O
2
(hydrogen
peroxide)/UV(ultraviolet) /O
3
(ozone) combined process was carried out when temperature was 30.0±0.6℃
and pH was 9.0±0.2. The influences of pre-treatment conditions, UV radiation intensity and wavelength
ratio, O
3
dosing rate and initial concentration on the removal efficiency of UDMH and COD were
researched. The results showed that the degradation rate of UDMH and COD increased as hydrogen
peroxide dosage, aeration gas velocity and time of pre-treatment process, UV radiation intensity and O
3
dosing rate increased. And the rate decreased as the initial concentration increased, and the rate of ozone
dosing and the intensity of ultraviolet radiation were the two factors that had the greatest impact on the
reaction rate. The removal efficiencies of UDMH and COD were 100% and 98.62% at the UDMH
concentration of 5000 mgꞏL
-1
for 60 min under the optimum conditions of the system. To conclude, there
were significant synergistic effects in this system. In the initial stage, the reaction was mainly led by
ultraviolet light while in the middle and late stages, the reaction was conducted and promoted by hydrogen
peroxide.
1 INTRODUCTION
UDMH waste water is usually produced in the
engine test, propellant transfer and rail tank cleaning
process, which is characterized by intermittent
generation, the composition, wide concentration
range, high organic content (Xia et al., 2013). The
main components of the waste water are UDMH, N-
nitrosodimethylamine, nitromethane, 1,1,4,4-tetra-
methyl-2-tetrazene, organic nitriles, aldehydes,
amines and so on (Liang et al., 2016). Most of which
belong to the above-mentioned chemical toxic
substances (
GBZ/T 229.2; GBZ/T 230), and are
carcinogenic, teratogenic and mutagenic. Its serious
impact on the environment has attracted more
people’s attention (Angaji and Ghiaee, 2015).
Advanced oxidation processes (AOPs) are a set
of chemical treatment procedures designed to
remove organic (and sometimes inorganic) materials
in wastewater by oxidation through reactions with
hydroxyl radicals (ꞏOH). According to the way
of ꞏOH generation and reaction conditions, it can be
divided into photocatalysis, sonochemical oxidation,
ozonation, electrochemical oxidation and Fenton/
Fenton-like methods.
These methods have been studied and applied in
the field of UDMH waste water treatment in recent
years. Jia Ying et al studied the photocatalytic
degradation of UDMH waste water by ZnO/Pd. The
experimental results showed that under natural light
conditions, the degradation rate of UDMH reached
80.5% and the removal rate of COD reached 75.7 %
(Jia et al., 2014). In the study on microwave-
enhanced Fenton method conducted by Zhang
Shujuan et al, the impact of different experimental
conditions on the degradation efficiency of UDMH
was studied. It was found that COD removal rate of
UDMH waste water was up to 98.4% under the
optimum conditions (Zhang et al., 2013). Jia Ying et
al studied the degradation of UDMH wastewater
through UV-Fenton method. In this study, they
compared the degradation efficiencies of the five
reaction systems designed. According to their results,
COD removal rate of UDMH waste water could be
up to 95.8% under the optimum conditions (Jia et al.,
2009).
92
Xu, Z., Huang, L., Chen, F. and Wu, Y.
Influencing Factors and Kinetics of Degradation of Unsym-Dimethylhydrazine Waste Water by H2O2/UV/O3 Process.
In Proceedings of the International Workshop on Environment and Geoscience (IWEG 2018), pages 92-98
ISBN: 978-989-758-342-1
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
These oxidation processes have complex
reaction systems and many refractory intermediates.
Ozonating technology is getting more and more
widely used in waste water treatment area,
especially in difficult biodegradable pollutants
treatment. This is because it has strong oxidizing
properties and rapid reaction rates and no secondary-
pollution will be produced (Lucas et al., 2010;
Cao
et al., 2016; Lee et al., 2016). However, due to the
low diffusion rate of ozone in water and high
operating costs, the application of it is subjected to
many restrictions. In recent years, people have
begun to study the combination of ozone and other
methods to improve the utilization of O
3
, such as
UV/O
3
, VUV/O
3
, H
2
O
2
/O
3
, H
2
O
2
/UV/O
3
and so on.
Based on the previous researches, the influence
of different factors on the degradation rate of
UDMH in H
2
O
2
/UV/O
3
system was analyzed in this
paper, and the optimum conditions of the system
were found out as well. The synergistic effect of this
system was researched to study the degradation
kinetics and provide a reference for the intermittent
treatment of UDMH waste water.
2 EXPERIMENT
2.1 Experimental Device and
Operating Conditions
H
2
O
2
/UV/O
3
combination process pilot test device is
shown in Figure 1. The aeration tank with a capacity
of 1.4m
3
is made of 316L stainless steel. And it can
be divided into three sections connected by flanges.
The diameter of the upper section is 500 mm and the
middle section is the reaction zone whose diameter
is 900 mm. In the tank, six UV amalgam lamps
(HANOVIA, 300W, radiation intensity:900μWꞏcm
-2
)
are installed, in which four have the wavelength of
185 nm and two the wavelength of 254 nm. Each
aeration tank has 12 aeration cylinders with a
diameter of 150 mm. Exhaust pressure of the air
compressor is 0.7 MPa. The volume flow and ozone
generation rate are 6.7 m
3
ꞏmin
-1
and 20 Nm
3
ꞏmin
-1
separately. The gas purity is equal to or higher than
90%(vol). The ozone generated by ozone generator.
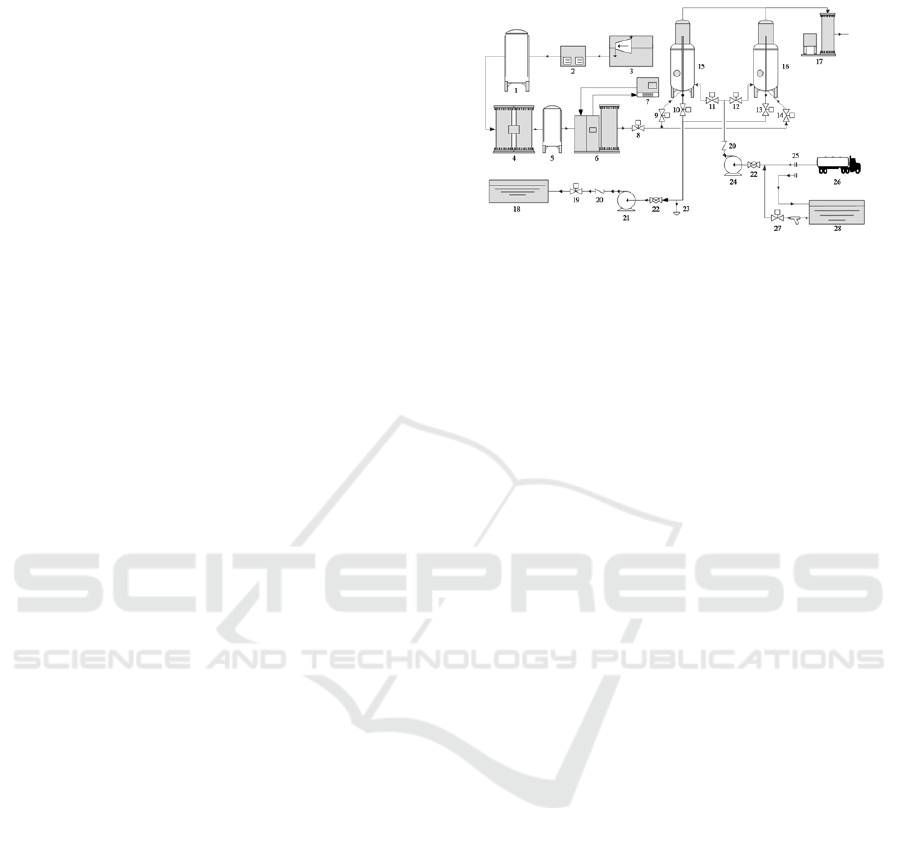
Figure 1: H
2
O
2
-UV-O
3
oxidization device and process.
1—gas holder; 2—air refrigeration dryer; 3—
compressor; 4—oxygen generator; 5—buffer tank;
6—ozone generator; 7—chiller;
8,9,10,11,12,13,14,19,27—electrical valve; 15,16—
reaction tank; 17—ozone destructor; 18—drainage
basin; 20—check valve; 21—drainage pump; 22—
manual valve; 23—sewage plug; 24—feed pump;
25—joints; 26—sewage lorry; 28—sewage reservoir
(Qingdao Guolin, CF-G-2-2kg type) is 100~120
mgꞏL
-1
and the yield is 2 kgꞏh
-1
.
2.2 Materials and Chemicals
GC-MS (Agilent 7890A-5975C); pH meter
(Shanghai Leici, PHSJ-3F); UV light meter
(Shenzhen Enci, UVX-254); HP8453E UV-Visible
spectrophotometer (Agilent Technology); solid
phase extraction column (Agilent Bond Elut-SCX).
UDMH (mass fraction was: UDMH, 99.59%;
water, 0.03%; dimethylamine, 0.06%; formaldehyde
dimethylhydrazone, 0.22%); hydrogen peroxide: 30%
(analytical grade, Shanghai Taopu chemical plant);
sodium hydroxide (analytical grade, Tianjin Beilian
reagent plant); methanol (analytical grade, Tritical
Company).
2.3 Experimental Process
The experimental process is shown below: (1) Pre-
treatment process. The hydrogen peroxide solution
(30% by mass) and saturated sodium hydroxide
(analytical grade) was added into the UDMH
sewage lorry or the sewage reservoir. The latter was
1% of the former by mass (
GB 6920-86
). After a
certain period of air aeration, the pre-treatment
process was completed. (2) Reaction process. The
oxidation process was carried out in sequence of
intermittent runs, in which about 2.0 m
3
waste water
was oxidized per cycle. After the pre-treatment,
Influencing Factors and Kinetics of Degradation of Unsym-Dimethylhydrazine Waste Water by H2O2/UV/O3 Process
93

UDMH waste water was added into aeration tank 1
by pump. Ozone was added after the generator ran
steadily, then the UV lamp was turned on. After a
certain time of reaction, it was switched to aeration
tank 2 and the process was repeated the whole
process was controlled by the programmable logic
controller (PLC).
Unless changed in the paragraphs below, the
reaction conditions were as follows concentration of
waste water C
UDMH
=5000 mgꞏL
-1
; reaction
temperature T=30.0 ℃, pH=9.0; pre-treatment gas
velocity V=1.5 m
3
ꞏmin
-1
; pre-treatment time t
pre
=6 h;
hydrogen peroxide dosage D
hyp
=47.2 gꞏL
-1
; UV
radiation intensity R=900 μWꞏcm
-2
; UV wavelength
ratio 60% 185 nm + 40% 254 nm; ozone dosing rate
D
ozone
=60 mgꞏ(Lꞏmin)
-1
; reaction time t
rea
=60 min;
UDMH removal efficiency and COD removal
efficiency: ratio of experimental value to initial
value(UDMH: 5000 mgꞏL
-1
, COD: 40000 mgꞏL
-1
).
2.4 Analytical Methods and Apparatus
PH was measured by pH meter (
GB 6920-86
) and
H
2
O
2
concentration by iodometric method (Gu and
Li, 2004), and COD by potassium permanganate
method (
GB 11914-89
), and radiation intensity by
UV light meter.
Waste water was measured by GC-MS, Agilent
7693A Autosampler; Column: DB-1701 Capillary
Column (30 m×0.25 mm×0.25 μm). Analysis
conditions: injection volume 1 μL; split injection;
split ratio 1:40; carrier gas flow rate 1 mLꞏmin
-1
;
inlet temperature 200 ℃; programmed temperature:
maintain at 50 ℃ for 5 minutes and rise to 160 ℃ at
the rate of 10 ℃ꞏmin
−1
and then maintain it for 3
minutes; EI ion source; mass scanning range:
29~280 amu; ion source temperature 230 ℃.
Waste water sample was pretreated by using
solid phase extraction column. Extraction conditions:
Solid phase extraction column was activated by 3
mL of methanol and balanced by 5 mL of deionized
water. Then 4 mL of acidic sample (pH was about 3
to 4) was taken and the flow rate was no more than 1
mLꞏmin
-1
. And then it was rinsed by 3 mL of
methanol and 3 mL of deionized water and dried for
1 minute. Finally, it was eluted with 2 mL of
saturated ammonia-methanol solution and collected.
3 RESULTS AND DISCUSSION
3.1 Influence of Pre-Treatment
(Hydrogen Peroxide)
3.1.1 Influence of Hydrogen Peroxide
Dosage
Taking the waste water of C
UDMH
=5000 mgꞏL
-1
for
example, the theoretical oxygen demand of UDMH
mineralization in 1.0 L waste water was 1.17 mol,
and the mass of hydrogen peroxide was 39.63 g.
That is to say, the theoretical dosage D
theory
was
132.08 gꞏL
-1
. When t
pre
was 6 h, the relation between
D
hyp
and the removal efficiencies of UDMH and
COD in waste water was shown in Figure 2.
Figure 2: Influence of H2O2 dosage on removal efficiency
of UDMH and COD.
As shown in the figure above, when D
hyp
was
47.2 gꞏL
-1
, the removal efficiencies of UDMH and
COD reached 76.5% and 63.4% separately. In the
experiment, H
2
O
2
was not added to fully mineralize
UDMH and that left in solution was 54.3% of the
dosage. The ratio of O
3
to H
2
O
2
was between 1.4:1
and 0.9:1, and the ratio of H
2
O
2
left to O
3
dosage
could be maintained between 1.0:1 and 0.5:1 after
the pre-treatment.
3.1.2 Influence of Aeration Gas Velocity and
Pre-treatment Time
Air aeration not only helped to
oxidize the
solution but also stirred it during pre-treatment. The
mass transfer of oxygen was a liquid membrane
control process. The liquid mass transfer coefficient
was improved as the gas velocity increased, thus
improving the shock mixing effect. The influence of
aeration gas velocity and pre-treatment time on
UDMH removal efficiency when t
pre
was 6 h~24 h
was shown in Figure 3.
IWEG 2018 - International Workshop on Environment and Geoscience
94
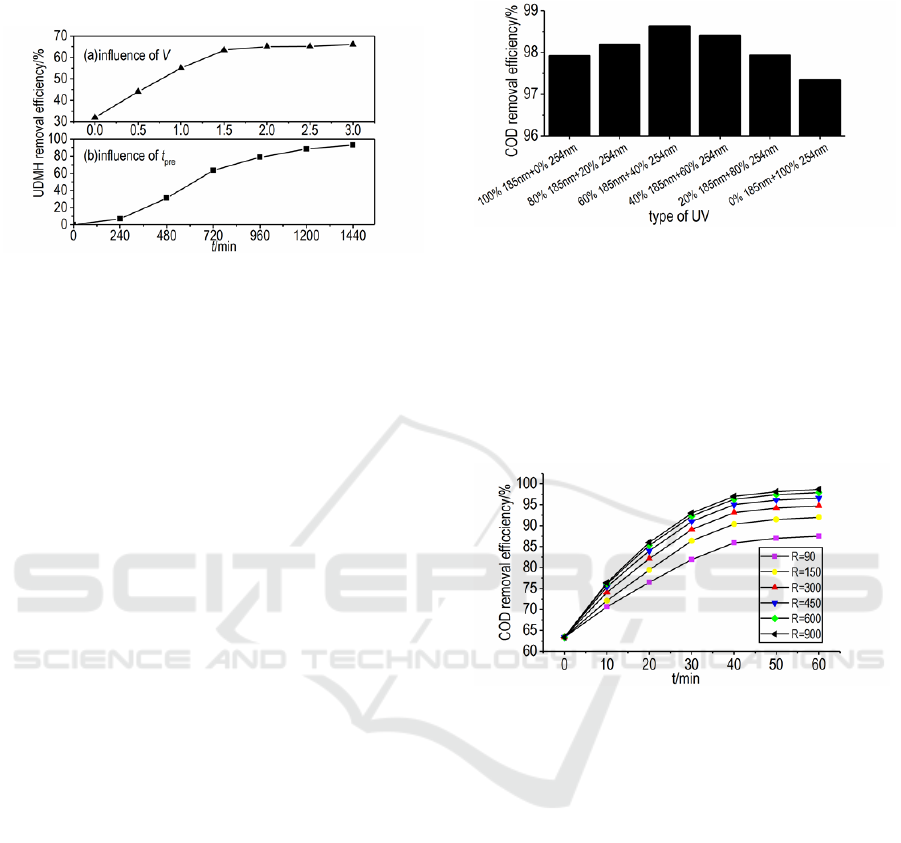
Figure 3: Influence of V and tpre on removal efficiency of
UDMH.
It can be seen from this figure that the removal
efficiency of UDMH increased as V and t
pre
increased, but the increase rate decreased due to the
oxidation capacity limitation of H
2
O
2
. H
2
O
2
was
oxidized through a chain reaction. Subsequent
products were easily generated during the pre-
treatment process through a series of chain
degradation reactions, which helped to prevent azide
compounds from forming in the combined oxidation
systems due to cross oxidization of UDMH. Limited
by costs and in consideration of the synergistic
effect of H
2
O
2
, O
3
and UV in the next step, the
optimal pre-treatment conditions were that V, t
pre
and
D
hyp
were 1.5 m
3
ꞏmin
-1
, 6 h and 47.2 gꞏL
-1
separately.
3.2 Influence of UV
3.2.1 Influence of UV Wavelength Ratio
Different UV wavelength ratios will produce
different synergies with O
3
and H
2
O
2
in the system
(Sekiguchi et al., 2007). In the experiment, UV light
sources of 185 nm and 254 nm were combined to
find out the optimal ratio. When t
rea
is 60 minutes ,
the relation between different UV wavelength ratios
and COD removal efficiency is shown in Figure 4.
As shown in the figure, when the ratio of UV of
185 nm to UV of 254 nm was 3:2, the COD removal
efficiency was the highest. As the ratio of UV of
single wavelength increased, the oxidation
efficiency gradually decreased, for the catalytic
efficiency of individual light was not as high as that
of UV and O
3
and H
2
O
2
.
Figure 4: Influence of UV type on removal efficiency of
COD.
3.2.2 Influence of UV Radiation Intensity
In order to maintain an uniform radiation, the UV
radiation intensity is regulated by adjusting voltage.
The influence of UV radiation intensity R on COD
removal efficiency is shown in Figure 5.
Figure 5: Influence of UV radiation intensity on removal
efficiency of COD.
In the figure, the removal efficiency of COD
increased as R increased, When R was 900 μWꞏcm
-2
,
COD removal efficiency could reach 98.62% after
60 min. In the oxidation system, O
3
and H
2
O
2
could
absorb UV to generate ꞏOH, while UV was the
necessary promoter of ꞏOH in the system. Therefore,
the higher the UV radiation intensity R is, the
photons released will be more. Besides, more free
radicals will be formed. Thus, it is necessary to keep
the intensity of the ultraviolet radiation at the
highest level that the amalgam lamp can achieve.
3.3 Influence of Ozone
In the absence of liquid flow, there was only gas
flow in the whole process. According to Henry's law,
the liquid mass transfer coefficient K
L
can be
derived as:
Influencing Factors and Kinetics of Degradation of Unsym-Dimethylhydrazine Waste Water by H2O2/UV/O3 Process
95

AK
dh
dC
Q)HRT(C
V
Q
d
σ
K
ozoneL
g
gozoneg
b
g
b
L
CC
(1)
Applying the boundary conditions (y=0,
C
g
=D
ozone
), the analytical solution of liquid ozone
concentration C
ozone
in steady state can be obtained:
)exp(
bbdgL
dL
bbdg
ozonegL
ozone
dVAKQKσ
hHRTAKKσ
dVAKQKσ
HRTQKσ
L
D
C
(2)
Where:
K
L
—
—
Liquid mass transfer coefficient,
m
ꞏs
-1
;
σ
—
—
The surface tension of water,
0.071 Nꞏm
-1
for tap water;
d
b
—
—
The average diameter of bubble,
m
;
Q
g
—
—
Intake air flow,
m
3
ꞏs
-1
;
V
b
—
—
Bubble rise speed,
m
ꞏs
-1
;
C
g
—
—
Gas - phase ozone concentration,
m
olꞏL
-1
H
—
—
Henry constant, molꞏ(Lꞏpa)
-1
;
R
—
—
U
niversal constants, 8.314
Jꞏ(molꞏK)
-1
;
T
—
—
Reaction temperature, K
C
ozone
—
—
Liquid ozone concentration,
m
gꞏL
-1
;
h
—
—
Bubble height, m;
A
—
—
Reactor cross-sectional area, m
2
.
If the self-attenuation coefficient of ozone can be
neglected (K
d
=0) after the steady state was reached,
equation (2) can be simplified as:
HRT
ozoneozone
DC
(3)
In other words, the ozone concentration in the
reaction system is determined only by D
ozone
, Henry
constant and T, regardless of the reactor size, inlet
flow rate, bubble particle size and other factors (Xu
et al., 2016).
Therefore, in the experiment, when Henry
constant and T are unchanged, only the impact of
D
ozone
on the degradation of COD in waste water
will be studied. It is shown in Figure 6.
In the combined oxidation system, UDMH and
its intermediates are mainly oxidized by ꞏOH. Under
UV irradiation, O
3
reacts with H
2
O to produce H
2
O
2
,
while H
2
O
2
absorbs UV to produce ꞏOH. O
3
can also
react with H
2
O
2
or H
2
O to generate ꞏOH. As D
ozone
increases, the amount of ꞏOH produced in the
system gradually increases as well, which results in
a higher COD removal efficiency.
Figure 6: Influence of Dozone on removal efficiency of
COD.
3.4 Influence of Initial Concentration
The influence of initial UDMH concentration on
COD removal efficiency is shown in Figure 7.
Figure 7: Influence of concentration of UDMH on
removal efficiency of COD.
As the initial concentration increased, the COD
removal efficiency decreased step by step, but the
absolute amount of oxidized COD increased at a
given time interval. When the initial concentration
of UDMH increased from 1500 mgꞏL
-1
to 10000
mgꞏL
-1
, the absolute amount of COD oxidized in 60
min changed from 4838.2 mg to 32132.1 mg. This
phenomenon may be resulted from the formation of
intermediates, which acted as a hydroxyl radical
scavenger at the beginning when the concentration
of UDMH was high, thereby reducing the rate of
oxidation reaction.
3.5 Synergistic Effect of the System
In the system, H
2
O
2
can be used as initiator and
accelerator for O
3
hydrolysis (Alkandari et al., 2016).
UV of 254 nm can lead to the decomposition of
H
2
O
2
(Minamidate et al., 2006). UV of 185 nm can
decompose O
2
in water to produce O
3
and promote
the hydrolysis of O
3
(Lekkerkerker et al., 2012).
IWEG 2018 - International Workshop on Environment and Geoscience
96

Specific synergies can be expressed briefly as
follows:
22223
OHOOHO hv
OH2OH
22
hv
OHOOHOH
32222
H
-
22
-
23
OOOHHOO
-
32
-
23
OOOO
2223
HOOOHOHO
22223
HOOOHOHO
OHHOOOHOH
222
products UDMHOH
productstesintermedia OH
3.6 Kinetics of Reaction
According to the data in Fig.2 to Fig.7, H
2
O
2
/UV/O
3
combined oxidation process of UDMH conforms to
the quasi-first order reaction kinetics process, and
the apparent quasi-first order reaction kinetics
equation applies to the degradation of UDMH
through the above process:
tkCC
obsUDMH
)/(ln-
(4)
C in equation (4) represent the concentration of
UDMH when reaction time is t, k
obs
is the quasi-first
order reaction kinetics constant. UDMH
concentration during the reaction, hydrogen
peroxide dosage, UV radiation intensity and ozone
dosage rate are the vital factors to degradation effect.
So, equation (4) can be expressed as:
d
c
ba
DRDCk
ozonehypUDMHobs
(5)
ε, a, b, c, d are constants and can be obtained
from the lg-lg diagram of the experimental data.
According to equation (4), (5) and experimental data,
the kinetic equation of the reaction can be written as:
194
7
.0
ozone
0509.0
0913.0
hyp
0021.0
UDMHobs
3-
107.3 DRDCk
(6)
From equation (6), it can be seen that the ozone
dosing rate D
ozone
and the UV radiation intensity R
have a great influence on the oxidation rate constant
of UDMH. With the increase in D
ozone
and R, the rate
constant increases almost linearly. The UDMH
concentration C
UDMH
has a little negative effect on
rate constants which is negligible.
4 CONCLUSIONS
(1) With a proper UV wavelength ratio, the
degradation rate of UDMH in waste water positively
correlated with the dosage of hydrogen peroxide,
aeration rate of pre-treatment, pre-treatment time,
ultraviolet radiation intensity and ozone dosing rate.
But the concentration of UDMH had little negative
influence on it.
(2) The optimum conditions were as follows: the
reaction temperature T=30.0 ℃, pH=9.0, the pre-
treatment parameters D
hyp
=47.2 gꞏL
-1
, V=1.5
m
3
ꞏmin
-1
, t
pre
=6 h, radiation intensity 900 μWꞏcm
-2
,
ratio of 185 nm ultraviolet light sources to 254 nm
ones 3:2, ozone dosing rate 60 mgꞏ(Lꞏmin)
-1
, the
reaction time 60 min the concentration of waste
water 5000 mgꞏL
-1
. UDMH and COD removal
efficiencies can reach 100% and 98.62% separately
under the conditions above.
(3) The H
2
O
2
/UV/O
3
combined oxidation system
has a more significant synergistic effect than
individual oxidation systems. The cause for the
synergic effect is the initial ultraviolet radiation on
the hydrolysis of ozone and hydrogen peroxide as
well as ozone decomposition resulted and promoted
by hydrogen peroxide in the reaction process.
(4) The H
2
O
2
/UV/O
3
combined oxidation process
conforms to the quasi-first order reaction kinetics
process. The kinetic equation can be written as:
1947.0
ozone
0509.0
0913.0
hyp
0021.0
UDMHobs
3-
107.3 DRDCk
, The ozone dosage rate and the UV radiation
intensity R have a great influence on the UDMH
oxidation rate constant.
REFERENCES
Alkandari H, Abdullah A M, Alkandari S 2016
Synergistic Effect of O
3
and H
2
O
2
on the Visible
Photocatalytic Degradation of Phenolic Compounds
Using TiO
2
/Reduced Graphene Oxide Nanocomposite
Science of Advanced Materials 5 739
Angaji M T, Ghiaee R 2015 Decontamination of
unsymmetrical dimethylhydrazine waste water by
hydrodynamic cavitation-induced advanced Fenton
process Ultrasonics Sonochemistry 257
Cao J, Xiong Z, Yuan Y 2016 Degradation of p -
nitrophenol (PNP) in aqueous solution by a micro-size
Fe0/O
3
process (mFe0/O
3
): Optimization, kinetic,
performance and mechanism RSC Advances 97 94467
GB 11914-89 water quality-Determination of chemical
oxygen demand-Dichromate method[S].
GB 6920-86 water quality- Determination of pH - Glass
electrode method[S].
GBZ/T 229.2 Classification of occupational hazards at
workplaces Part 2 [S] 2010
GBZ/T 230 Classification for hazards of occupational
exposure to toxicant [S] 2010
Gu Yingying, Li Dan 2005 Study on the superhardness
Influencing Factors and Kinetics of Degradation of Unsym-Dimethylhydrazine Waste Water by H2O2/UV/O3 Process
97

mechanism of Ti-Si-N nanocomposite films: Influence
of the thickness of the Si3N4 interfacial phase J
Hunan Institute of Science and Technology 3 55
Jia Y, He Y N, Liang F H 2014 Photocatalytic degradation
of UDMH wastewater with nano particles of ZnO/Pd
Chinese Journal of energetic materials 4 554
Jia Y, Li Y, Zhang Q Y 2009 Thermal decomposition and
combustion performance of azidonitramine gun
propellant containing RDX Chinese Journal of
energetic materials 3 365
Lee Y, Gerrity D, Lee M 2016 Organic Contaminant
Abatement in Reclaimed Water by UV/H
2
O
2
and a
Combined Process Consisting of O
3
/H
2
O
2
Followed
by UV/H
2
O
2
: Prediction of Abatement Efficiency,
Energy Consumption, and Byproduct Formation
Environmental Science & Technology 7 3809
Lekkerkerker K, Knol A H, Altena L 2012 Serial
ozone/peroxide/low pressure UV treatment for
synergistic and effective organic micropollutant
conversion Separation & Purification Technology 44
22
Liang M, Li W, Qi Q 2016 Development of japonica
Photo-Sensitive Genic Male Sterile Rice Lines by
Editing Carbon Starved Anther Using CRISPR/Cas9
RSC Advances 7 5677
Lucas M S, Peres J A, Puma G L 2010 Treatment of
winery wastewater by ozone-based advanced
oxidation processes (O
3
, O
3
/UV and O
3
/UV/H
2
O
2
) in a
pilot-scale bubble column reactor and process
economics Separation and Purification Technology 3
235
Minamidate W, Tokumura M, Znad H T 2006
Photodegradation of o-cresol in water by the H
2
O
2
/UV
process J Environmental Science & Health Part A 8
1543
Sekiguchi K, Jeong J, Sakamoto K 2004 Removal of
Organic Gaseous Contaminants by the Reaction of
Labile Species Using Different Wavelengths of
Radiation from a Single UV Source Earozoru Kenkyu
19 188
Xia B L, Wang L, Liu Y 2013 Hydrothermal Synthesis
and Gas Sensing Properties of α-Fe
2
O
3
Hollow
Microspheres and Nanorods Advanced Materials
Research 343-344 303
Xu Z L, Zhang L Q, Zhao B 2016 Degradation of
Unsymmetrial Dimethylhydrazine Waste Water by
Hydrogen Peroxide Enhanced UV-Ozone Process
Chinese Journal of Energetic Materials 12 1168
ZHANG S J, CHEN X J, WU W E 2013 Degradation of
Unsymmetrical Dimethylhydrazine with Microwave
Enhanced Fenton Chinese Journal of energetic
materials 4 455
IWEG 2018 - International Workshop on Environment and Geoscience
98
