
Thermal Decomposition Mechanism of Metal Xanthate to
Metal Sulfide Nanoparticles in Ammonia Solution
H L Lian, Q Shen, Y J Fan, L M Wu and Z X Sun
*
School of Chemistry and Chemical Engineering, University of Jinan, 250022 Jinan,
China
Corresponding author and e-mail: Z X Sun, sunzx@ujn.edu.cn
Abstract. Synthesizing metal sulfide nanoparticle by thermally decomposing metal xanthate
is a facile method; however, the proposed mechanism rationalized by Chugaev reaction has
not been experimentally proved. Herein we report our experimental evidence to elucidate the
reaction mechanism. In this study, ZnS and CdS nanoparticles of a few nanometers in size
were prepared by thermal decomposition of a single source precursor of metal xanthates in
ammonia solution at temperature as low as 90°C. The particle size and crystallinity were
characterized using XRD techniques. The decomposition mechanism studied by UV spectra
in combination with GC-MS and FTIR is fund to be a nucleophilic elimination reaction with
main products of MS nanoparticles, alkanols, manothiocarbonate, carbonyl sulfide and
dixanthogen. Olefin, a main product of Chugaev reaction, is not detected in this process,
which suggests that the thermal decomposition mechanism of MX
2
to MS is not that of
Chugaev reaction.
1. Introduction
Metal sulfide such as ZnS and CdS semiconductor nanoparticles (NP) as advanced materials has
attracted much research attention due to their wonderful size-dependent tunable optical properties
[1]. As a semiconductor compound, ZnS or CdS and their mutual core shell structure possesses
photoluminescence (PL) and electroluminescence (EL) and have applied as sensors and lasers [2],
light-emitting diodes when doped [3, 4], solar cells [5], catalysts [6]. In past years, synthesizing ZnS
or CdS using single source precursor of metal chalcogenide compound has been explored, the results
is encouraging. O’Brien’s group reported their results for the synthesis of CdS nanoparticles [7].
Efrima’s group synthesized a series of metal sulfide NP, the thermal decomposition mechanism of
the chalcogenide precursor was theoretically rationalized using the Chugaev reaction [1,8]. This
mechanism is accepted by some researchers when they prepare NiS NP [9]. However no
experimental evidence of the formation of olefin was provided in all these studies. On the other hand
different mechanism for thermal decomposition of single precursor of metal chalcogenide was also
proposed [10], in which the decomposition products were thiourea, hydrogen sulfide and solid metal
sulfide nanoparticles. Apparently people’s concerns over the thermal decomposition of single
precursor of metal chalcogenide are inconsistent and a generalized mechanism has not yet been
commonly accepted. We used a single source precursor of cadmium xanthates with variable carbon
chain length in an ammonia solution to synthesis size tunable CdS nanoparticles, and the
268
Lian, H., Shen, Q., Fan, Y., Wu, L. and Sun, Z.
Thermal Decomposition Mechanism of Metal Xanthate to Metal Sulfide Nanoparticles in Ammonia Solution.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 268-275
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

experimental results didn’t support the hypothesis based on Chugaev reaction [11].
Neither did a
published paper using metal xanthate as a precursor to prepare highly luminescent quantum dot of
metal sulfide [12].
In order to understand the thermal decomposition mechanism of the metal xanthate in ammonia
solution further, we used zinc or cadmium xanthate as a single source precursor to prepare ZnS or
CdS NP and the process is inspected respectively by UV, FTIR and GC –MS spectroscopic
techniques.
2. Experimental
2.1. Material and methods
Zinc acetate, potassium hydroxide, potassium n-heptylxanthate, ethanol, acetone, petroleum, aqueous
ammonia, ether and other chemicals used in the present study were all reagent grade and all water
used in the experiments was deionized distilled water.
The ZnS and CdS powders produced were characterized by X-ray diffraction (XRD) using a
Bruker D8-Advance X-ray diffractometer employing Cu Kα radiation (wavelength 1. 54 Å). A
continuous mode was used for collecting data from 10° to 80° of 2θ at a scanning rate 0.1°•S
-1
.
A Shimadzu UV-2450 UV-visible (UV-vis) spectrophotometer and a Bruker VERTEX-70 FTIR
was used to carry out optical measurements and to follow the thermolysis of the samples. Samples
were placed in quartz cuvettes (1 cm path length).
GC-MS analyses were performed on a Thermo Electron DSQ quadrupole mass spectrometer
connected directly to a Thermo Electron Focus gas chromatograph and to an autosampler AS 3000
(Thermo Electron, Dreieich, Germany). A fused-silica capillary column Optima-17 (15 m × 0.25 mm
i.d., 0.25 μm film thickness) from Macherey-Nagel (Düren, Germany) was used. The gas over
reaction liquid was collected using a 1 mL injecting syringe and measurements were performed by
selected-ion monitoring (SIM) of m/z 240 for d0-ethanol and m/z 245 for d6-ethanol with a dwell
time of 50 ms for each ion. The following oven temperature program was used with helium as the
carrier gas at a constant flow rate of 1 mL min
−1
: 1 min at 70 °C, then increased to 180 °C at a rate of
30 °C min
−1
, and to 280 °C at a rate of 70 °C min
−1
; the oven temperature of 280 °C was held for 1
min. Interface, injector and ion source were kept at 280 °C, 200 °C and 250 °C, respectively.
Electron energy and electron current were set to 70 eV and 100 μA, respectively, for electron-capture
negative-ion chemical ionization (ECNICI) with methane as the reagent gas at a flow rate of 2.4 mL
min
−1
.
2.2. Synthesis of ZnX
2
and CdX
2
After dissolving 0.02 mol K (CH
3
(CH
2
)
6
OCS
2
) in distilled water, 100 mL solution (containing 0.013
mol zinc acetate) was drop wise added. Then a white precipitate appeared. After the reaction
completed, the product was centrifuged at 7000 rpm for 10 min, rinsed three times with a ethanol
solution (the volume ratio of water and ethanol was 3:1) to yield purified Zn(CH
3
(CH
2
)
6
OCS
2
)
2
.
2.3. Synthesis of ZnS or CdS by the thermal decomposition of ZnX
2
or CdX
2
.
In a flask containing 60 mL ammonia solution and placed in an oil bath heated up to 90℃, 300mg
Zn(CH
3
(CH
2
)
6
OCS
2
)
2
was added to the flask with stirring, after refluxing for 8 hours to decompose
the ZnX
2
completely. The solution was cooled gradually at room temperature and then the
supernatant separated from the solid particles. The deposits was collected by centrifugation at 7000
rpm for 10 min and washed three times with ethanol and water.
3. Results and discussions
The X-ray diffraction results are shown in Figure 1.
Thermal Decomposition Mechanism of Metal Xanthate to Metal Sulfide Nanoparticles in Ammonia Solution
269
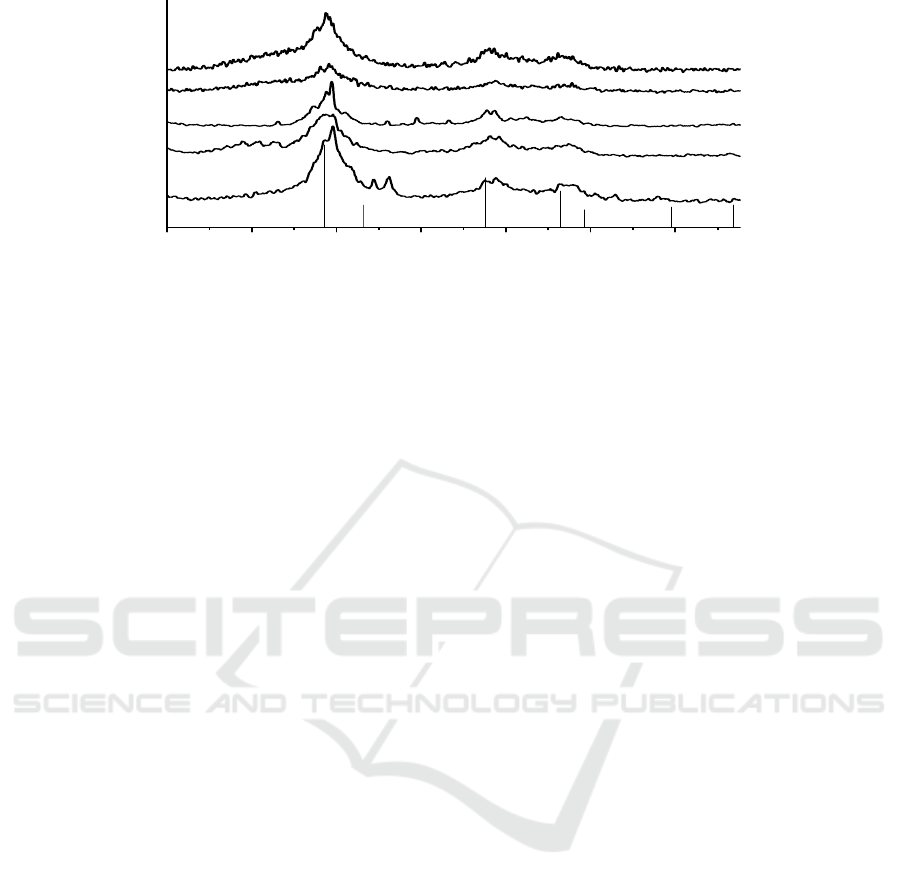
0203040506070
e
d
c
b
121
112
103
102
100
110
2 θ/°
111
220
311
101
Δ Δ Δ
102
a
∗∗
Figure 1. XRD data for zinc sulfide prepared by decomposing zinc n- heptylxanthate in alkaline.
Solution of ammonia or sodium hydroxide at different reaction time at 90
°C.(a: 5 h; b: 9 h; c: 15 h;
in ammonia solution at pH about 13.4). (d: yellowish solid; e: 9 h; in sodium hydroxide solution at
pH about 12.2). (* is NaZnO
2
; △ may be from heptyldixanthogen)
Diffraction peaks at 2 θ values between 25.0~35.0°, 45.0~50.0°, and 55.5~60.5° can be clearly
seen in Figure 1 curve a, which is indexed as the (111), (220), and (311) plane of zinc sulfide
respectively. However, the (100), (101), (102), (103) plane of wurtzite ZnS appears in the curve c
after reacting for 15 hours, indicating the presence of hexagonal wurtzite ZnS as well. When solution
pH increases less than 13, a yellowish solid can be yield. In order to explore the mechanism, we did
another parallel experiment, in which we use sodium hydroxide as the nucleophilic agent to attack
the most electrons deficient C-S bonds in ZnX
2
to yield zinc sulfide. The XRD results are shown in
Figure 1. curve e. The curve d is yellowish solid produced in ammonia and zinc sulfide in sodium
hydroxide solution, respectively. Diffraction peaks at 2 θ values between 25.0~35.0°, 45.0~50.0°,
and 55.5~60.5°, identified as the (111), (220), and (311) peaks of zinc sulfide can be clearly seen.
However, some weak peaks at 2 θ values between 15.0~25.0°, 33.2~35.5°, 36.0~38.0° can also be
seen. The peaks at 15.0~25.0° may be identified as the dixanthogen’s diffraction peaks. And the
others can be identified as the (102), (121) peaks of NaZnO
2
which could be produced by the side
reaction during the thermal decomposition process of ZnX
2
in sodium hydroxide solution. The
crystallite size was calculated here using Scherrer’s equation:
d = Kλ/(βcos θ) (1)
where d is the crystal size, the X-ray wavelength, the broadening of the diffraction peak and θ the
diffraction angle. The sizes of the crystallites determined according to the broadening of (111)
diffraction plane of the ZnS crystal are 3.2, 3.8, 4.3 nm for a, b, c and 3.1 nm, 3.9 nm for d, e
respectively. Obviously, the particle size of formed ZnS increases progressively with increasing
reaction time. XRD data for cadmium sulfide prepared by decomposing variable cadmium xanthates
in ammonia solution has been reported in a previews paper of ours [11], which proved the thermal
decomposition products were size variable CdS NPs, therefore not repeat here.
In order to find out the thermal decomposition mechanism of zinc and cadmium xanthates in
alkaline solution, the solution samples at different reaction time were carefully analyzed using UV
technique, the measurement results of UV spectra are shown in Figure 2, in which the UV absorption
spectra of ZnX
2
and CdX
2
in ammonia solution at 90°C are measured with varying time.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
270

200 225 250 275 300 325 350
Absorbance (a.u.)
wavelengh/nm
A
226
301
0 min
30 min
120 min
300 min
480 min
200 225 250 275 300 325 350
Absorbance (a.u.)
wavelength/nm
0 min
25 min
75 min
125 min
185 min
365 min
395 min
B
Figure 2. UV absorption spectra for zinc sulfide and cadmium sulfide prepared by decomposing
ZnX
2
or CdX
2
with different reaction time at 90°C (where from top to bottom denote 0 min, 30 min,
120 min, 300 min and 480 min in Figure 2A for ZnX
2
, and 0 min, 25 min, 75 min, 125 min, 195 min,
365 min and 395 min in Figure 2B for CdX
2
respectively).
From Figure 2 we can see the absorbance peak at 301 nm is increasing in the first 30 min for both
ZnX
2
and CdX
2
suspension, indicating the concentration of xanthate is increasing i.e. xanthate is
releasing from the metal xanthate to solution. With increasing reaction time, the peak at 301 nm
decreased progressively in both ZnX
2
and CdX
2
suspensions, suggested the xanthates released from
metal xanthate were consuming gradually together with the formation of the metal sulfide
nanoparticles, meanwhile the absorbance peak at 226 nm shows a few wavelengths red shift. After
closely evaluating the red shift we found out that the final peak actually comes from the combination
of three peaks i.e. a peak at 222 nm, a peak at 226 nm and a peak at 238 nm after simulating. As a
typical example, Figure 3 shows the simulated peaks for UV spectra for CdX
2
after reacting for 365
min at 90°C in ammonia solution.
200 210 220 230 240 250 260
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
222
226
238
235
experim ental curve
fitted cu rv e
Absorbance
w avelength/nm
Figure 3. Simulated peaks for UV spectrum for CdX2 after reacting for 365 min at 90°C in ammonia
solution (the solid line denotes the recorded UV spectrum; dashed lines denote simulated peaks). The
four curve fitted sub-peaks (dashed lines) shown resulted in the sum of squares (SS) of 0.00051.
The curve fitted sub-peak was located at 222 nm, 226 nm and 238 nm respectively. The peak at
222 nm and the peak at 238 nm belong to the absorbance from monothiocarbonate and dixanthogen
respectively [13]. In the same way, we simulated the UV spectra for ZnX
2
after reacting 30, 120, 300
and 480 min and for CdX
2
after reacting for 25, 75, 125, 185 and 395 min respectively at 90°C in
ammonia solution, the results are presented in Table 1. From Table 1 we can see that with increasing
Thermal Decomposition Mechanism of Metal Xanthate to Metal Sulfide Nanoparticles in Ammonia Solution
271

reaction time, the peak intensity at 226 nm attributed to xanthate increased in the first 30 min and
then decreased continuously in line with that at 301 nm shown in Figure 2. Apparently the variations
of the peak at 301 nm and simulated peak at 226 nm show the same trend, implying xanthate is one
of the thermal decomposition products of metal xanthate. At the same time, the simulated peak at 238
nm attributed to dixanthogen increased at the first 300 min and then shown somewhat decreasing,
indicating dixanthogen is one of the thermal decomposition products. However, the simulated peak at
222 attributed to monothiocarbonate shown some different phenomena for ZnX
2
and CdX
2
system. In
ZnX
2
suspension, it increased at the first 5h and then stabilized. In CdX
2
system, however, it
increased at the first 2h and then decreased irregularly, which may indicate to some extent different
intermediate thermal decomposition process for ZnX
2
and CdX
2
system. These phenomena may
reveal that the monothiocarbonate may be an intermediate thermolysis product, which then goes
through further decomposition.
Table 1. Variations of UV absorbance in ZnX
2
or CdX
2
suspension during the thermal decomposition process.
precursor Time/min Wavelength/nm Sum of
squares
222 226 238
ZnX
2
0 --- 0.35 ---
30 0.08 0.45 0.10 0.043
120 0.15 0.37 0.17 4.33E-4
300 0.32 0.04 0.19 1.03E-5
480 0.31 0 0.17 5.57E-5
CdX
2
0 -- 0.36 --
25 0.10 0.57 0.15 0.00862
75 0.15 0.53 0.27 0.0472
125 0.26 0.25 0.38 5.83E-4
185 0.13 0.22 0.47 0.010033
365 0.14 0.19 0.54 5.1E-4
395 0.06 0.12 0.53 0.00132
Whenever dealing with the thermolysis of xanthate, people naturally tend to consider it as a well
known Chugaev reaction. Efrima’s group has rationalized the thermal decomposition mechanism of
various metal xanthates in alkylamine solution using Chugaev reaction.
1
In order to test the
applicability of Chugaev reaction in explaining the reaction mechanism in our system, we made great
effort to find out the evidence of olefin, as the main thermal decomposition product of Chugaev
reaction is olefin and if it is there, it should be easily identified by gas chromatography in
combination with mass spectroscopy (GC-MS) and FTIR measurements. However, the
measurements results didn’t show any evidence of olefin, but different degradation products of
xanthate (supporting information, Figure S1-S2), which is consistent with the results of our UV
spectroscopic measurements. From the results of FTIR measurements, we can find a peak at wave
number of 2053 cm
-1
, due to the asymmetric stretching vibration from the carbonyl sulfide. From the
results of GC-MS measurements, the peak at retention time of 1.428 min is assigned as that from CS
2
,
in which 76, 44, 32 corresponds respectively to the mass spectrum of CS
2
, CS and S. From the mass
spectra at retention time of 1.428 min, we can also see the mixed mass spectra of air and carbonyl
sulfide with m/z=60, 44, 32, 28, 16, 12, m/z=60 is COS and m/z=44, 32, 28 can be attributed to the
degradation products of COS and their mixture with CO
2
, O
2
and N
2
. The experimental results of this
GC-MS measurement can evidence the existence of COS, which is in consistence with the results of
FTIR measurements.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
272
In order to confirm what else the yellowish solid shown in Figure 1 contains, we measured its
FTIR and UV spectra, the results are shown in Figure 4.
4000 3600 3200 2800 1600 1200 800 400
Transmittance (a.u.)
wavenumber/cm
-1
29602925
2850
1605
1302
1207
1090
1050
720
720
1207
1050
1460
1380
a
b
A
200 225 250 275 300 325 350
Absorbance (a.u.)
wavelength/nm
in ethanol
in water
240
279
301
206
238
c
d
B
Figure 4. IR and UV absorption spectra for zinc sulfide prepared by decomposing ZnX
2
at 90°C
(where (a) ZnX
2
; (b) the yellowish solid on the surface of ZnS; (c) yellowish solid in ethanol
solution; (d) yellowish solid in water solution).
In Figure 4A, the curve ‘a’ is ZnX
2
and the curve ‘b’ is the yellowish solid sample. In the range
from 3000 to 2800 cm
-1
, characteristic bands for carbon-hydrogen vibrations are seen. The peaks
around 2960 and 2925 cm
-1
are the asymmetric methyl (CH
3
) and methylene (CH
2
) vibration bands,
respectively; and the bands around 2870 and 2850 cm-1 are the corresponding symmetric vibrations.
The two sets of absorption bands between 1360 and 1390 cm
-1
as well as between 1420 cm
-1
and
1480 cm
-1
are due to C-H bending modes and appear in a similar position, to a greater or lesser extent,
in all of the FTIR spectra shown in Figure 4A. The band at 1200 cm
-1
is mainly due to the
asymmetric stretching vibration of C-O-C, and the band between 1064 and 1021 cm
-1
has a strong
involvement of the asymmetric S-C-S stretch [14, 15]. According to the curve ‘b’, the peaks at 1605
and 1090 cm
-1
are the characteristic bands for O-heptyl monothiocarbonate group (R-O-C(O)-S-).
The set of absorption band at 1605 cm
-1
is due to the stretch vibration of carbonyl group (C=O) and
the peaks around 1190 cm-1 is the asymmetric stretch vibration of -C-O-C- group. The peaks at 1302,
1207 and 1050 cm
-1
are the characteristic bands for n-heptyldixanthate.
Figure 4B is UV absorbance spectra for the solution of the yellowish solid sample in water and in
ethanol solution, respectively. As we know that n-heptyldixanthogen is hydrophobic and can easily
dissolve in ethanol and hardly in water. Clearly the peaks at 240 nm and 279 nm in ethanol solution
and 238 nm in water of n-heptyldixanthogen can be seen [12]. The peak at 206 nm attributes to CS
2
produced by the decomposition of the remained n-heptylxanthate.
According to the Chugaev reaction, Pradhan and Efrima proposed a mechanism including
decomposition products of metal sulfide, carbonyl sulfide, olefins and xanthic acid.1 However, the
decomposition products in our system are xanthate, monothiocarbonate, carbonyl sulfide and
dixanthogen, which suggested that Chugaev reaction cannot account for the decomposition
mechanism of metal xanthate in this system.
According to the decomposition products observed we suggest that the decomposition of MX
2
via
three steps, which is schematically illustrated in Figure 5. Our proposed mechanism was partly
supported by some evidences proposed by Jones and Woodcock in 1982 [16], when they tried to
understand the reaction mechanism of xanthate in mineral flotation.
Thermal Decomposition Mechanism of Metal Xanthate to Metal Sulfide Nanoparticles in Ammonia Solution
273

M
S
O
R
S
Z
OH
-
R
O
S
M Z
OH
-
S
OHO
R
S
+
R
O
S
M
S
S
-
S
-
O
R
S
+MS
R OH
+
C
O
S
3
1
2
4
R
O
S
S O
R
S
S
MS surface
5
Z =
S
O
R
S
R =
M = Z
n
, Cd
Figure 5. The thermal decomposition mechanism of ZnX
2
and CdX
2
in ammonia solution.
Firstly, the NH
3
, OH
-
might attack the O-C-S
2
Zn group, moving negative charge from OH
-
toward
the C=S bond. Then the S-M-Z group estranged from MX
2
and formed Z-M-S
-
, and the remaining
was R-O-C(S)-OH. Secondly, the groups can decompose sequentially. As the group R-O-C(S)-OH
breaks down; O-C-S and n-hepthanol emerged. The Z-M-S
-
group further decomposes into metal
sulfide and n-heptylxanthate anion. However, the reaction will continue if solution pH is lower than
13. At last the dixanthogen will be formed by the reaction shown in Figure 5. Double groups of
ROC(S)S
-
can be oxidized into n-heptyl dixanthogen on the surface of the zinc sulfide.
4. Conclusions
Following conclusions can be drawn from this investigation.
1. The size of cubic MS particles is between 3 nm and 4.1 nm by a thermolysis method of a single
source precursor of MX
2
.
2. The decomposition process of MX
2
is a nucleophilic reaction and NH
3
and OH
-
act as powerful
nucleophilic agents in the reaction.
3. The decomposition products contain zinc sulfide, xanthate, O-heptyl monothiocarbonate,
carbonyl sulfide, heptanol and dixanthogen.
Acknowledgments
Financial support from Chinese Natural Science Foundation (No. 51274104; No. 50874052),
National Basic Research Program of China (No. 2011CB933700) is gratefully acknowledged.
References
[1] Pradhan N, Katz B and Efrima S 2003 J. Phys. Chem. B 107 13843
[2] Tanne J, Schäfer D, Khalid W, Parak W J and Lisdat F 2011 Anal. Chem. 83 7778
[3] Fang X M, Roushan M, Zhang R B, Peng J, Zeng H P and Li J 2012 J. Chem. Mater. 24 1710
[4] Yin X J, Xie G H, Peng Y H, Wang B W, Chen H, Li S Q, Zhang W H, Wang L and Yang C
L 2017 Adv. Funct. Mater. 27 1700695
[5] Yu X Y, Liao J Y, Qiu K Q, Kuang D B and Su C Y 2011 ACS NANO. 5 9494
[6] Zong X, Han J F, Ma G J, Yan H J, Wu G P and Li C 2011 J. Phys. Chem. C. 115 12202
[7] Nair P S, Radhakrishnan T, Revaprasadu N, Kolawole G and O’Brien P 2002 J. Mater. Chem.
12 2722
[8] O'Connor G L and Nace H R 1953 J. Am. Chem. Soc. 75 2118
[9] Alam N, Hill M S, Kociok G, Zeller M, Mazhar M and Molloy K C 2008 Chem. Mat. 20 6157
[10] Jung Y K, Kim J I and Lee J K 2009 J. Am. Chem. Soc. 132 178
[11] Zhang W M, Sun Z X, Hao W, Su D W and Vaughan D 2011 Mater. Res. Bull. 46 2266
[12] Todescato F, Chesman A, Martucci A, Signorini R and Jasieniak J J 2012 Chem. Mater. 24
2117
[13]
Shankaranarayana M L and Patel C C 1961 Can. J. Chem. 39 2590
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
274

[14] Ihs A, Uvdal K and Liedberg B 1993 Langmuir 9 733
[15] Fredriksson A and Holmgren A 2007 Colloids Surf. A. 302 96
[16] Jones M H and Woodcock J T 1983 Int. J. Miner. Process. 10 1
Thermal Decomposition Mechanism of Metal Xanthate to Metal Sulfide Nanoparticles in Ammonia Solution
275
