
Experimental Study on Electrochemical Machining of Conical Micro-
Holes
Weimin Gan
1
, Ye Zhu
1,2
, Bo Xu
1
, Yang Chen
1
and Xiangzhi Wang
1
1
Jiangsu Key Laboratory of Non-Traditional Machining
,
Changzhou Institute of Technology, Changzhou 213002,CHN
2
School of Mechanical Engineering, Changzhou University, Changzhou 213164
Keywords: Tube electrode; simulation analysis; conical hole; ECM.
Abstract: Micro holes with internal features are widely used as nozzle of engine nowadays, which are usually required
to be with high aspect ratio and shape accuracy, as well as good surface quality. In order to solve the
machining problem of the engine nozzle, a small taper hole was studied by electrochemical machining
which has online machining tube cathode .By processing machining model and doing simulation of flow
field and electric field, analyze the influencing factors in the experiment. Finally, the law of the feed speed,
the voltage and electrolyte concentration and taper of hole are studied. A good conical micro-hole was
worked out and taper error is 0.46 °.
1 INTRODUCTION
Mechanical components tend to be miniaturized and
refined, and are widely used in engines, micro-
electromechanical systems, precision instruments
and other fields. Among them, the structure of the
conical hole is complex and difficult to process. The
conventional machining technology cannot achieve
the general adoption of special processing
technology. For example, the micro-tapered cavity is
machined by electric spark or laser [1], and then the
surface quality of the side wall is improved by
abrasive flow polishing. However, in the EDM
process of micro holes, the electrode loss is serious,
the machining accuracy is difficult to ensure, and
there are defects such as surface microcracks in laser
processing [2]. The surface processed by the
abrasive flow is improved compared with laser
processing, but the residual stress is difficult to
eliminate [3].Electrochemical machining has the
advantages of no loss of cathode, good surface
quality, and no residual stress. Prof. Li Yong of
Tsinghua University processed micro-holes with
smaller taper in the ECM test by changing the
voltage between the electrodes, the duty ratio, and
the feed rate, and achieved better results. Good
effect, import deviation is 3μm and cone angle error
<0.1° [4]. XuShiyu of Xi'an University of
Technology successfully processed tapered holes by
formulating a layered electrolytic milling process
[5]. Nanjing University of Aeronautics and
Astronautics adopts a compound feed electrolytic
machining process to machine large taper holes with
clear contours and stable machining [6].
The electrochemical product of micro-conical
hole is difficult to discharge in the electrochemical
machining. In order to eliminate manufacturing and
secondary assembly errors, set up a micro-hole
processing device, on-line machining conical tube
electrodes. With the use of electrolyte injection
technology and rotation of cathode, through the
experimental study the influence of factors such as
cathode feed rate, processing voltage and electrolyte
concentration on the processing results.
2.INFLUENCE OF
ELECTROLYTIC PROCESSING
PRECISION OF TAPER HOLES
From the basic laws of electrolytic machining,
namely Faraday's law and Ohm's law, it can be
deduced that the dissolution rate of the anode during
electrochemical processing in formula (1).

v
a
=ηωi=
(1)
In the equation (1) where v
a
is the anode phase
dissolution rate; η is the electrolyte current
efficiency; i is the current density; ∆b is the end
clearance; ω is the volume electrochemical
equivalent; к is the electrolyte conductivity; U
R
is
the ohmic pressure drop between poles . This
formula reflects the parameters of the
electrochemical machining and is the theoretical
basis for analyzing the forming law of
electrochemical machining.
In electrochemical machining, the size of the
machining gap and its variation are the main sources
of error in machining. The machining gap is affected
by many factors such as electric field, flow field,
temperature and electrochemical characteristics [7].
According to electrolytic processing end surface
equilibrium gap formula(2).
b= (2)
Fully differentiate it to get the gap d∆b:
= [ (3)
Formula (3) shows that the use of small gaps can
reduce the amount of change in the gap, thereby
increasing the gap of electrochemical machining.
Figure 1 Schematic diagram of electrochemical machining
of tapered tube electrode.
As shown in Figure 1, at any one of the cathode
feeds z, it is again available:
(4)
Formula (4) shows that when the conical cathode
continuously feed, end clearance ∆b affect the
important factors of the precision of the tapered hole
processing. From formula (3) ,the processing
voltage, feed rate and electrolyte concentration
affect the end of the tapered hole Gap, thus affecting
the processing stability and machining accuracy of
the tapered hole.
3.PREPARATION OF
EXPERIMENT
3.1 Device of Experiment and Online
Machining of Cathode
This experiment was based on a CNC engraving and
milling machine. An L-shaped stainless steel plate
was placed on the lower end of the spindle, and the
processing device was connected to feed the taper
hole up and down. As shown in Fig. 2, the high-
pressure pipe joint leads to the electrolyte and flows
from the liquid storage cavity into the cathode of the
tapered pipe to form a liquid. As the diameter of the
conical pipe is small, a 0.8 mm seal ring is inserted
into the liquid storage cavity to avoid high pressure.
The electrolyte overflows to ensure that the
electrolyte flow is normal during processing. The
drill chuck can hold 0.15mm-3mm diameter and it is
not easily deformed. A carbon brush is placed in the
conductive block, and the cathode and the power
line are connected to form a path. The DC motor
drives the cathode through the belt to rotate during
processing and the spindle feed speed of CNC
machine tool can reach 1μm/s, which can meet the
requirements of the micro-hole machining.
Figure 2 Device of ECM.

The manufacture of tool electrodes in the electro-
processing of cone-shaped holes is very important.
Turning and grinding of the tube electrodes can
easily lead to bending of the front section of the
cathode and leave marks on the surface, failing to
meet the test requirements. The cone electrode can
be obtained by electrochemical processing with a
conical block. The processing device is as shown in
FIG. 3. At this time, the tool electrode is connected
to the anode of the power supply, the cone
correction block is connected to cathode, and the
motor drives the cathode to rotate at a relatively high
speed. The current sensor is used to observe the
processing current, and control the removal amount
by changing the processing voltage and processing
time. In the experiment, two types of materials that
copper and stainless steel were used to make the
cathode of the tapered tube. The surface of the
resulting copper electrode was seriously pitted due
to the use of nitric acid. Sodium electrolyte is not
suitable for this type of material. Since the stainless
steel is resistant to electrolyte corrosion and the
hardness of the material is high, the stainless steel
electrode obtained in FIG. 5 has a uniform taper and
a good surface quality, and can be used as a forming
cathode for the electrolysis of a tapered hole.
Figure 3 Device diagram for on-line cathode fabrication.
Figure 4 Copper conical tube electrode.
Figure 5 Stainless steel tapered tube electrode.
3.2. Analysis of Flow Field Simulation of
Conical Tube Electrode
Assuming that the fluid is constant, incompressible,
ideal, the loss of energy due to electrolyte
temperature changes and temperature differences
during processing is negligible, and the flow follows
mass and momentum conservation equations.
Figure 6 Three-dimensional model diagram of electrolyte
flow channel.
Figure 7 Velocity distribution of conical surface.

In electrochemical machining, in order to satisfy
the assumption of a steady distribution of the flow
field, and to better remove the electrochemical
products at the surfaces of cathode and anode to
reduce the concentration polarization near the
electrodes and to make the liquid flow uniform, the
processing gap should be turbid. Flow state
electrolyte, electrolyte flow rate v0 should meet [5].
(5)
In the formula (5),v is the viscosity coefficient of
water, which is used here as an alternative to the
viscosity coefficient of the electrolyte. The
electrolyte temperature is 25°C in the experiment,
and the viscosity coefficient is 0.89 10^(-6)
m2/s[5];Dh is the hydraulic diameter, that is, the
hollow diameter of the tube electrode, which is
substituted into the formula.
5.1m/s (6)
From the formula (6), the stability of the
electrochemical processing can be guaranteed only
when the flow rate of the electrolyte in the
processing gap is at least 5.1 m/s. From the taper
tube electrode processing gap flow velocity
distribution chart, when the inlet pressure is 0.5Mpa,
the flow velocity in the processing gap is greater
than 5.1m/s, and the velocity distribution is uniform
stable. A very small number of processing areas
outside the emergence of low flow rates, so need to
choose import pressure parameters over 0.8Mpa.
3.3. Electric Field Characteristics of
Cone Tube
Assuming that the electrolyte is isotropic, according
to the electric field theory, it can be seen that the
potential distribution conforms to the Laplace
equation and its equation is
(7)
Boundary conditions of anode surface
is
: (8)
The boundary condition of cathode surface is:
(9)
In the formula,φ is the potential of each point
in the electric field, generally φ = φ (x, y, z); U is the
surface potential of the anode; n is the normal
coordinates of the anode surface everywhere; θ is the
angle between cathode feed rate and the normal
direction of anode; is the current efficiency; η
0
is
the current efficiency at θ=0; i0 is the current density
in the normal direction of the anode surface at θ=0; к
is the electrolyte conductivity.
Because (10) (11)
The boundary between the processed material
and the electrolyte is
(12) (13)
Electric field simulation uses 14% sodium nitrate
solution whose conductivity is 8.7 (S/m) and the
anode material is 0Cr18Ni91 (304 Stainless steel),
where the processing voltage 4v. From the figure 8,
the current density is gradually weakened along the
material to the micro-electrode direction, and the
maximum value appears on the interface between
the processed material and the electrolyte, and is
unevenly distributed along the boundary surface.
The maximum value is
, the
minimum value appears on the contact surface
between the conical tube electrode and the
electrolyte. The value is
. The
current density is an important parameter for
electrochemical machining. Generally, with the
voltage increasing, the current density increases, and
the bottom surface processing effect is better. [6]
However, the side current density is too large, the
material removal amount increases, and the taper
increases. The faster the machining speed, the less
stray corrosion on the side, the smaller the taper, the
closer to the taper of the forming cathode.
Figure 8 Distribution of electric field density.
4. EXPERIMENT OF PROCESS
PARAMETERS
In order to study the influence of process parameters
on the precision of the electro-processing of conical
holes, a single-factor comparison test was conducted
on the feed rate, processing voltage, and electrolyte
concentration, keeping the other processing
parameters unchanged. And taper effect of the law.
The unilateral lateral clearance ∆s and both sides of
the taper were used as evaluation indexes. In the test,
the taper hole was measured with an ultra-depth
digital microscope and the hole diameter and hole
taper were measured. Since the gap between the top
and bottom side of the taper hole was not uniform,
the three-point calculations were taken at the inlet
end, the middle end, and the bottom end. Unilateral
side of the average side of the gap ∆ s, the formula is
below.
(14)
In the formula (14), D is the diameter of the
upper surface of the conical hole obtained by
electrochemical machining, whose unit is μm and d
is the diameter of the tube electrode. The main
parameters are shown in Table 1.
Table 1 Selected main parameter tables.
Category Main parameters
Electrolyte
composition
NaNO
3
Electrolyte
temperature (°C)
25
Electrolyte pressure
(MPa)
0.8
Cathode taper (°) 20
Cathode speed
(r/min)
200
Power type Pulse power
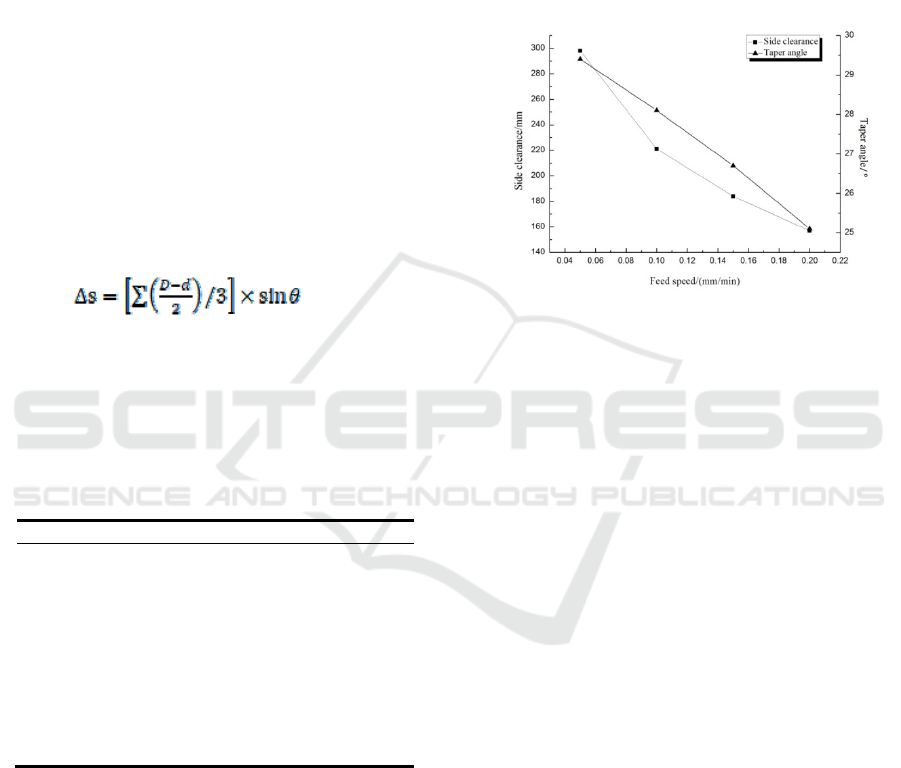
4.1 The Influence of Feed Rate on Taper
of Taper Hole
Select the sodium nitrate electrolyte mass fraction of
14%, the processing voltage which is 4v, change the
processing speed to obtain the test data, and get the
effect of the feed speed on the unilateral gap and
taper. The processing quality is the basis of the
electrochemical machining of the tapered hole.
Appropriately increasing the feed rate helps to
increase the processing efficiency and is essential for
improving the productivity of electrolytic
processing. As shown in the figure 9, as the feed rate
increases, the side gap gradually decreases, the
secondary corrosion time on the side becomes
shorter, and the inlet diameter becomes smaller.
However, when electrolysis is performed at a
relatively fast feed rate, a short-circuit phenomenon
is likely to occur, and the taper on the side surface is
not uniform.
Fig. 9 Influence of feed speed on side clearance and taper.
4.2 Effect of Processing Voltage on Taper
Cone Drilling
From the analysis of the electric field characteristics,
it can be seen that the higher the voltage, the higher
the current density at the end face and the side, and
the more serious the diffusion of the tapered hole,
resulting in a larger taper, an increase in the taper
error with the cathode, and a lower processing
accuracy.
For machining smaller conical holes, choose
lower tool cathode feed rate, preferentially
0.1mm/min, 14% sodium nitrate solution for testing.
As can be seen from figure 10, as the processing
voltage is increased, the unilateral gap on the side
becomes larger, the larger the hole diameter, the
more severe the corresponding stray corrosion, and
the greater the taper of the workpiece. Therefore, in
the electrolysis process, the choice of processing
voltage is extremely important. However, as the
processing voltage decreases, the material removal
rate and the machining efficiency are lower, and the
radial clearance increases. Therefore, a reasonable
processing voltage must be used.

Fig. 10 Influence of processing voltage on side clearance
and taper.
4.3 Influence of Electrolyte
Concentration on Taper
Select 10%, 14% and 18% sodium nitrate electrolyte
as a comparative test, and found that 10% sodium
nitrate solution in electrochemical processing, the
number of short circuit more, and the surface shown
in Figure 10, select 18% sodium nitrate solution
processing When the processing speed is adjustable
to 0.3mm/min, the machining efficiency is better,
but the taper is larger and the machining accuracy is
not high. With proper increase of the electrolyte
concentration, ion concentration become higher and
effect of ions in the processing area become
stronger. Because the localized effect of the
electrochemical material removal reaction get
smaller, the stray current increases removing of
material. Therefore, in order to improve the
processing accuracy, it is necessary to reasonably
limit the electrolyte concentration. Experiments have
shown that by choosing a smaller electrolyte
concentration and processing voltage, the lateral
clearance can be reduced. The processing gap
decreases as the electrolyte concentration decreases.
Therefore, the selection of a low concentration of
electrolyte is advantageous for forming a small
processing gap. The excessive reduction of the
concentration of sodium nitrate solution leads to a
decrease in the electrical conductivity and hence to a
decrease in the current density. This results in a
rapid decrease in the removal rate of the workpiece
and a short-circuiting and burning of the workpiece.
A B
Fig. 11 Bottom map of mass fraction 10% A and 14% B
sodium nitrate solution.
5. OPTIMIZATION OF TEST
RESULTS
Based on the above experimental research and
analysis, the optimization parameters were selected
for electrolytic processing of conical tube electrodes.
The NaNO
3
solution with a mass fraction of 14%
was selected for the test, the processing voltage was
4V, and the feed rate was 0.1mm/min. Taper
difference between the two sides of 0.46 ° taper, to
meet the processing needs. The error in the
machining results shown in Fig. 8 is small, and it
can be seen from the partial enlargement that the
accuracy of the shape is high, and both sides show
good localization and processing capability.
500
μm
Figure 12 Conical hole profile view.
6. CONCLUSIONS
(1) An experimental study on the production of two
different materials of the tube electrode by on-line
electrolytic machining has been carried out, and it
has been found that the stainless steel conical tube
electrode can be used as a cathode for processing a
tapered hole.
(2) By establishing the mathematical model and
processing model of the electrolysis machining of
the tapered tube electrode, the influence factors and
electric field characteristics of the processing
precision of the tapered tube electrode are analyzed,

and the parameter test is verified in the ECM
experiment.
(3) Set up a conical tube electrode tester to
analyze the effects of processing voltage, feed rate,
and electrolyte concentration on the unilateral gap
and taper. Select NaNO
3
solution with a mass
fraction of 14%, the processing voltage which is 4V,
and the feed rate which is 0.1mm/min and process a
better tapered hole whose taper error is smaller,
import roundness is better and cone burns is less.
ACKNOWLEDGEMENTS
The research of this subject has been funded by the
Jiangsu University Natural Science Research Project
(No. 15KJA460002),Jiangsu Postgraduate Practice
Innovation Plan (No. SJCX17_0732), National
Natural Science Foundation of China(Grant No.
51705040) and Natural Science Foundation of
Jiangsu Province, China (Grant No. BK20150255).
We also extend our sincere thanks to all who
contributed in the preparation of these instructions.
Thank you very much!
REFERENCES
1. L Yong ,HRuiqin. Micro Electrochemical Machining
for Tapered Holes of Fuel Jet Nozzles[J] ProcediaCirp
, 2013 , 6 :395-400.
2. Kraus Martin, Collmer Stephan, Sommer Steffen,
DausingerFriendrich. Micro drilling in steel with
frequency-doubled ultrashort pulsed laser radiation[J].
Journal of Laser Micro,2008 , 3 (3) :129-134 .
3. Huang Ying, Zhang Xiangjun, Li Yong. Extrude
honing abrasive slurry and its honing experiments for
fuel jet nozzle[J]. China SurfaceEngineering,2011,24
(5) :68-72.
4. G Liu , Y Li , Q Kong , H Tong. Research on
ECM process of micro holes with internal
features.Precision Engineering , 2017 , 47 :508-515.
5. QiLu. Fundamental Research on compoundFeeding
Precision ECM of Nozzle Taper Hole
[D].NanjingUniversity of Aeronautics and
Astronautics, 2016.
6. XuShiyu. Microelectrochemical processing of a
certain engine nozzle spin chamber [D].Xi'an
University of Technology, 2013.
7. DU Pan, ZHAO Jianshe, YU He etc. Effect
ofCompound Feed on Electrolytic Processing of
Conical
Holes[J].ElectromachiningandMolding,2017(02):23-
27.
8. He Yafeng, Lu Wenzhuang, GanWeimin et al.
Numerical study of micro-pit electrolytic machining
based on the coupling of electric field and flow
field[J]. ChinaMechanical
Engineering,2016,27(10):1365-1370.
