
70% Ethylated and Non-Ethylated Swab: A Trace Evidence Recovery
Method and Usefulness in Spectacles Forensic Evidence
Simon Martin Manyanza Nzilibili
1,3
, Muh. Abduh Dwi Putra
1
, Ahmad Yudianto
1,2,4
1
Forensic Science Program, Post Graduate School, Airlangga University, 4-6 Airlangga Rd., 60286, Surabaya - Indonesia.
2
Forensics and Medico-legal Department, Faculty of Medicine, Airlangga University, Surabaya – Indonesia
3
Ministry of Health, Community Development, Gender Elderly and Children, Dodoma – Tanzania.
4
Human Genetic Laboratory, Institute of Tropical Disease, Airlangga University, Surabaya – Indonesia.
Keywords: Ethyl, Spectacle, Trace Evidence, 70% Ethylated swab
Abstract: Trace evidence recovery has been studied to involve a number of methods with the inclusion of swabbing
methods. In swabbing, different fluids have also been proposed with minimal highlight to ethylated swabbing
(prepared from ethanol). Through the use of swabbing fluid at 70% dilution (70% ethylated swab) run
simultaneously with distilled water swab, the findings discovered were that 70% ethylated swab was found to
concentrate large amount of DNA sample (1421µg/ml), twice the amount recovered by distilled water swab
(654.5µg/ml). In terms of purity of recovered DNA; 70% ethylated swab presented nearly similar purity with
distilled water DNA pellet (ethylated swab yielded 1.219 purity ratio while distilled water swab yielded
1.176). Electrophoresis DNA molecule migration displayed multiple bands in contrast with 70% ethylated
swab from positive to negative electrode. This shows the presence of both small and larger sized DNA
fragments. Distilled water swab displayed one deep band near negative terminal electrode. Thereby; the study
finding analysis illustrates and suggests that 70% ethylated swab is a useful and strong method in recovering
trace DNA evidences for successful DNA profile establishment. In addition, spectacle is prospective a
potential harbor of trace DNA sample for forensic investigation.
1 BACKGROUND
Recovery of micro- to macro-Deoxyribonucleic acid
(DNA) trace evidence is studied to involve adhesive
tape, forceps, and vacuum methods (Fisher et al.
2007; Ah Van Oorschot et al. 2010). Swab method is
also discussed and presented to work properly and is
far better, while moist in recovering such trace
evidences (van Oorschot et al. 2003; Ah Van
Oorschot et al. 2010; Jack Dillon, Debra Figarelli,
David Sylvester 2009; Oregon State Police 2015;
Puritan 2016; Adamowicz et al. 2014). It is opted and
used as moisturising fluid in case of remains of high
and efficacy determinant. To explore the
effectiveness, the experimental studies done have
explored the success of ethyl moist swabbing, though
most still recommend water moistening option (van
Oorschot et al. 2003; Raymond et al. 2008). Thus, in
the DNA retrieval study; 95% ethanol swab
(Slrichantrawonq et al. n.d.) was found to be suitable
to recover DNA at a high yield than distilled water
(Hildebrand et al. 2004), in which a 25% ethylated
alcohol swab yielded the most compared to those with
50% concentration and distilled water swab.
Emphasizing essence to these innovations is the
maximized retention of minute natured sample
residing on swab after recovery against loss or blow-
away (Fisher et al. 2007). Contrary to a noted good
use as swab fluid, ethyl on the other hand is presented
as a decontaminant and lyses catalyst (Gršković et al.
2013), which means that it has a destructive effect in
opposition to recovery usefulness. Despite of
substantial trials on alcohol fluid, specified ethyl
percentage which is effective to be used in swabbing
is yet to be established. This study therefore dedicated
furthered investigation on a 70% ethylated swab
supply on spectacle evidence convinced by reasons
below;
1.1 Why 70% Ethylated Alcohol Swab?
The percentage of alcohol on treatment of biological
sample has a direct relationship. On DNA extraction
and preservation usage, 70% ethyl has evidenced a
312
70 .
DOI: 10.5220/0007541903120316
In Proceedings of the 2nd International Conference Postgraduate School (ICPS 2018), pages 312-316
ISBN: 978-989-758-348-3
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

flexible treatment of biological samples which allow
morphological exploration afterwards (Oswald
2007). This is in contrast to high or low percentage in
respect to a volatile, drying blow-away, non-flexible,
and degradation-prone performance. In addition;
compared to other alcohol groups such as
isopropanol, ethanol is highly precipitous that
resuspend DNA pellet easily. The insolubility of
DNA molecules is catalysed by forming H-bonds
with water during isolation (decrease hydration
ability of water to DNA) – reduced decaying. Lower
dielectric leads DNA to aggregate and concentrate
with cations below lighter molecules under phenol-
chloroform extraction (Brennan 2017; Zumbo 2013).
Referring to the percentage used in the reference
above, ethylated alcohol swab envision has a special
use in recovering DNA trace evidence. 70% ethyl
alcohol swab is optimised for consideration as
explained earlier. This ground prompted the
exploration of the usefulness of 70% ethylated swab
through admission of spectacle as useful and reliable
suggested source of trace biological evidence based
on Locard’s and Kick’s contact and silent witness
respective principles. Appreciation of 70% ethyl
swab and admission of spectacle, in addition to
normally referred evidences such as clothes, knife,
vehicles, firearms, bedding, food, condoms, lip
cosmetics, wallets, jewellery, glass, skin, bullet,
paper, cables, windows and door lockers/handle,
stones and watch (Ah Van Oorschot et al. 2010)
broaden exhibits. Either in recovery of such micro or
macro exhibits like hair, dust, soil, glass particles,
fluids, touched surfaces, clothes (Fisher et al. 2007)
as forensic evidence, limited information is on the
spectacles as potential source of biological trace
evidence. Apart from compilation through literature
reviews of the established useful properties of the
ethyl alcohol in forensic evidence; this study
complemented the findings used to recover trace
DNA from spectacles especially through the use of
70% saturation. Aggregation of these information
potentiate establishing special 70% ethyl swab for
trace evidences swabbing recovery as reported in this
study compared to most recommended water swabs.
2 MATERIALS AND METHODS
The article paralleled literature reviews on available
studies of ethylated alcohol swab application or
usefulness and experimental authentication of
spectacle evidence. Experimental content was
conducted at the University Human Genetic
Laboratory involving two biological samples
swabbed from two different spectacles: one by a 70%
ethyl swab and the other by a distilled water swab.
2.1 Sample Recovery
Samples to determine the usefulness and application
of ethylated swab was obtained from two participants
who voluntarily gave their spectacles after being
given a clear understanding of the study purpose.
From two spectacle evidences, DNA trace biological
sample was recovered by a separate swabbing under
one swabbing direction and surface without
repetition. The two swabs used were sterilised and
cotton-made. One swab was a readymade 70%
ethylated which swabbed one spectacle, and the other
was a dry swab which was moistened by 1cc of
distilled water and swabbed the second spectacle. The
process was immediately followed by a tube soaking
of spectacle swabbed swabs into 2 different tubes
filled by 4cc distilled water overnight to allow down
settling of DNA biological traces recovered for DNA
analysis.
2.2 DNA Extraction
The extraction process proceeded with removing of
upper most fluids while retaining down settled sample
solution. Then, 0.5cc of each sample was isolated in
a sterile centrifuge tube; pipetted with 1cc of DNAZol
(Invitrogen, ThermoFisher Scientific, Waltham, MA,
USA), and vortexed and incubated for 15 minutes.
Then, it was vortexed with 0.2cc of Chloroform
(Merck KGaA, 64271 Darmstadt, Germany)
followed with a centrifugation at 8,000 rpm for 10
minutes. Separated supernatant was obtained into
eppendorf with isopropanol 1cc (EMSURE®, Merck
KGaA, 64271 Darmstadt, Germany), 15 minutes
incubation, and centrifugation at 12,000 rpm for 10
minutes then with care followed by the discarding of
supernatant fluid again, leaving settled and
concentrated pellet. The pellet was washed with 0.5cc
of 70% ethanol (EMSURE®, Merck KGaA 64271
Darmstadt, Germany), and it then underwent 15
minutes of centrifugation at 12,000 rpm for 5
minutes, which again was followed by the removal of
supernatant through Chen et al. (2010) as well as
Chomczynski et al. (1997) protocols. Finally, 50µl of
distilled water resuspended formed DNA pellet for
spectrophotometer and electrophoresis.
~
70
313

2.3 Spectrophotometer Measurements
Spectrophotometry measurement was done in order
to establish concentration and quality parameters of
70% ethylated swab in reference to distilled water
swab, as well as the potential extent of spectacle
evidence in yielding significant biological sample for
DNA. Using Ultraviolet-visible Spectrophotometer
(UV-1601, PC, Shimadzu, Japan), DNA
Concentration was determined by absorbance reading
at 260nm and 280 in UV-1061, while DNA Purity
was given by Optical Density (OD) OD260/OD280
ratio, refer Table 1.
2.4 Electrophoresis
Electrophoresis of acrylamide gel method was opted
due to sensitivity even to minute sample without
Polymerase Chain Reaction (PCR) (purposely to find
out if both 70% ethylated swab and spectacle
referenced to water swab can yield interesting results
without polymerase amplification). The gel was
prepared by a 3cc acrylamide reagent (Sigma-
Aldrich) mixed with 8cc Tris-borate-EDTA (TBE) -
0.5x (Promega Corporation, Madison, USA). Then,
Temed (Sigma-Aldrich) 20µl followed with 200µl
ammonium perisulfate solution (Sigma-Aldrich)
under homogenization cycles at 100 Volts for 60
minutes (Figure 3).
3 RESULTS AND DISCUSSION
3.1 70% Ethyl swab recovery method
According to literature, alcoholic swab has
exemplified usefulness in varied percentages. As
found from this study, the alcoholic swab with a
concentration of 70% was reasonably examined and
presented to substitute and establish a useful ethylated
swab potential for maximizing recovery of trace
biological evidence. Compared the two swabs used,
the 70% ethyl alcoholic swab and distilled water
swab, the findings presented closer results in
spectrophotometer measurement (Table 1) but quite
different in electrophoresis band contrast (Figure 3).
Concentration reading was measured almost three
times in 70% ethylated swab compared to distilled
water swab as per Figure 1. This concentration gives
the interpretation that 70% ethylated swab recovers
more DNA samples compared to possible amount
able to be recovered by distilled water swab. In
forensic profiling analysis, this interpretation gave a
meaning to the usefulness of increased probability
and assured the recovery of an adequate amount of
sample from targeted evidence of traces that is
potential to enable successful profiling results during
experimentation.
Table 1: Concentration and Purity of Spectacle DNA
evidence under ethylated and non-ethylated swab
Sample
Code
Absorbance
260 nm
Absorbance
280 nm
DNA
Concentration
(ng/ul)
DNA
Purity
Distilled
Water
Swab-A 0.187 0.159 654.5
1.17
6
70%
Ethylate
d Swab-
B
0.406 0.333 1421
1.21
9
In deducing the purity measurement, the
generated purity increased confidence for usage of
ethylated swab. Estimated chances of increased
degradation and destruction of genetic materials as
anticipated through previous few reported
applications in decontamination pose a contrary
scenario. According to the studies; ethyl being as
destructive agent forecasted expectation that this
study also significantly generated a lowered purity by
the fact of its destructive ability (micro-organisms
discussed similar to structure of traces). Purity of the
70% ethylated swab recovered sample was nearly
similar above distilled water swab. Despite the fact
that both 70% ethylated swab and distilled water
swab were below the recommended limits of purity
(1.6-2.0) (Table 1), the findings suggested that use of
modern extraction would purify to acceptable limits.
The nature of results measured project useful pellet
(of acceptable limit) with agreed contribution of
effective recovery of ethyl swab.
Figure 1: Concentration of DNA extracted from 70% ethyl
and distilled water swabbed spectacle.
DNA was recovered with ethyl swab concentrated
DNA amount more than twice as high (1421µg/ml) as those
concentrated by distilled water swab (654.5µg/ml). The
ethyl method concentrated DNA amount due to its ability
to recover even the stickiest sample traces as much as
possible.
Distilled
Water
Swab (A)
32%
Ethylated
Swab (B)
68%
DNA Concentration (ng/ul)
314
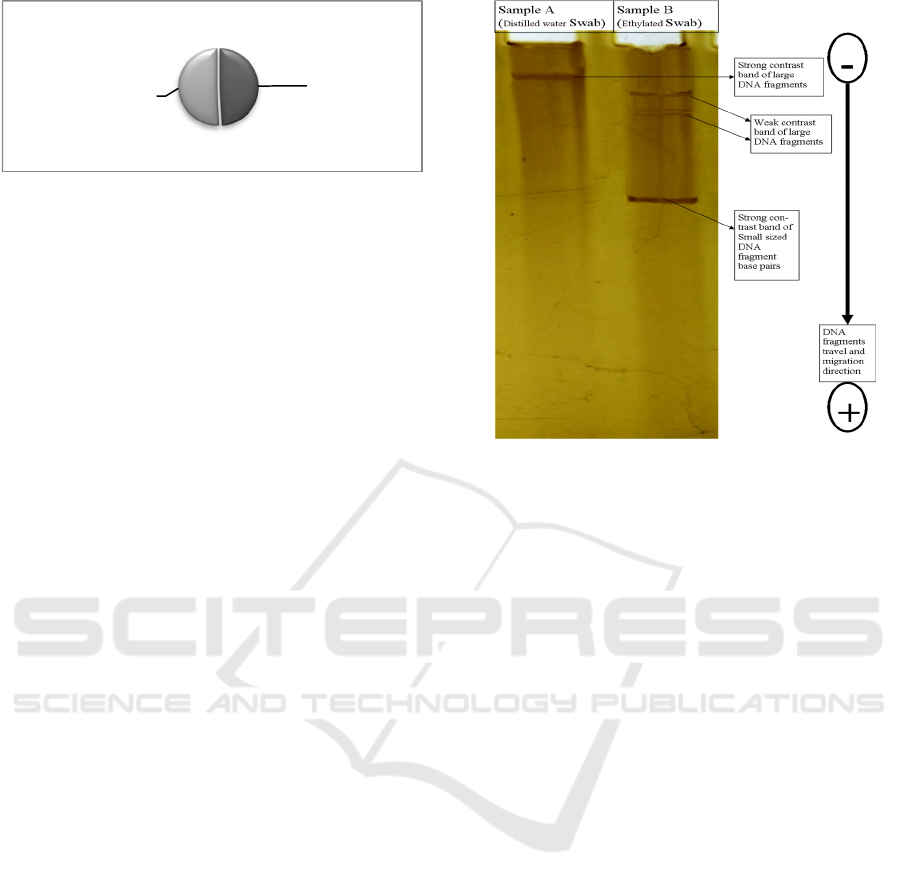
Figure 2: Purity of DNA extracted from 70% ethyl and
distilled water swabbed spectacle.
Purity was assessed and compared to sample extracted by
water swab/ 70% ethyl swab was measured to have pure
DNA in a similar ratio. Purity of DNA was well-detected
outside of quality range. Thus, this similar quality (though
a bit high in ethyl) complemented the significance of the
use of 70% ethylated swab.
3.2 Electrophoretic Reaction
Electrophoresis DNA band contrast appeared to both
swabs. Migration of DNA fragments from negatively
charged electrodes to positive was established in
different numerous band level especially to sample of
the 70% ethyl swab as presented in Figure 3. This
band contrast portrayed the size, length and strength
of the DNA extracted from these two kinds of swabs
(ethylated and water swabs). As discussed in
concentration and purity above (Table 1), DNA
obtained through 70% ethylated swab was with nearly
similar purity but higher collected amount as
portrayed in Figure 1. This information implis that
both swabs yielded pure DNA capable to be analyzed
in electrophoresis, as shown in Figure 2. The meaning
is that DNA has successfully been electrophorised as
full charged fragments and migrated to appropriate
contrast level. However, from the displayed bands,
ethylated swab contrasted more bands compared to
water swab. This suggests that 70% ethyl swab
recovered more amount of DNA sample with various
strength and size leading to a differed migration of
which small-sized fragments were lighter and
migrated faster with contrast level closer to Anode
electrode as referred in Figure 3. The longer and
larger sized DNA fragments recovered in ethylated
swab and water swab appeared to contrast closer to
cathode electrode due to slower migration of charged
fragments.
Figure 3: Electrophoresis band contrast of DNA sample
recovered through 70% ethyl and distilled water swabbed
spectacle evidences.
This interpretation suggests three things. Firstly,
both swabs (70% ethylated and distilled water)
recovered a significant amount, but ethylated swab
recovered more significant minute traces evidenced
by different band contrasts and even being
concentrated more at lower level (closer to positive
end). Secondly the purity of DNA sample recovered
by ethylated swab was higher with excess
concentration compared to water swab. Thirdly,
minute and increased extraction of sample was of
useful quality as being able to be profiled on
electrophoresis even without PCR primer
amplification.
3.3 Spectacle
As other evidences were found at crime scene through
this study, spectacle was evaluated to useful and
potential evidence able to be used as source of trace
DNA for profiling as a result of contact from humans
that used it before. Through a well-established
recovery method, spectacle exhibit can significantly
contribute to the logged and harbored amount of
DNA in contacted sample from specific individuals
used before.
4 CONCLUSION
DNA spectrophotometer and band visualization
contrast depicted and suggested 70% ethylated swab
Distilled
Water
Swab …
Ethylated
Swab (B)
51%
DNA Purity
70
315

to be a useful and strong method that recovers large
and enough samples for DNA profile establishment.
As for the reasons stated above in signifying
conduction of this study, 70% of ethyl is in the
manner recommended due to it being a flexible
percent that tolerates further morphological treatment
of DNA sample from recovery and let them in for a
temporal storage before processing in the laboratory.
The study has also brought attention to the
consideration of spectacle as potential source of DNA
sample either found at crime scene for criminal
linkages or to purposed forensic inquiry for
investigative profiling.
REFERENCES
Adamowicz, M.S. et al., 2014. Evaluation of methods to
improve the extraction and recovery of DNA from
cotton swabs for forensic analysis. PLoS ONE, 9(12),
pp.1–18.
Ah Van Oorschot, R., Ballantyne, K.N. & Mitchell, R.J.,
2010. Forensic trace DNA: a review. Investigative
Genetics, 1, p.14.
Brennan, J., 2017. What Does Ethanol Do in a DNA
Extraction? Sciencing.com. Available at:
https://sciencing.com/ethanol-do-dna-extraction-
8336005.html [Accessed January 14, 2018].
Chen, H. et al., 2010. Evaluation of five methods for total
DNA extraction from western corn rootworm beetles.
PLoS ONE, 5(8).
Chomczynski, P. et al., 1997. DNAzol: A reagent for the
rapid isolation of genomic DNA. BioTechniques, 22(3),
pp.550–553.
Fisher, B.A.J., Fisher, D.R. & Kolowski, J., 2007.
Forensics demystified, McGraw-Hill.
Gršković, B. et al., 2013. Effect of ultraviolet C radiation
on biological samples. Croatian medical journal, 54(3),
pp.263–71. Available at:
http://www.pubmedcentral.nih.gov/articlerender.fcgi?
artid=3692334&tool=pmcentrez&rendertype=abstract.
Hildebrand, D.P. et al., 2004. DNA Sampling from the
Trigger and Handgrip of Discharged Firearms.
Jack Dillon, Debra Figarelli, David Sylvester, W.T., 2009.
Collecting DNA Evidence at Property Crime Scenes.
Dna Initiative, pp.1–30.
van Oorschot, R. et al., 2003. Are you collecting all the
available DNA from touched objects? International
Congress Series, 1239(C), pp.803–807.
Oregon State Police, 2015. Physical Evidence Manual,
Oswald, N., 2007. The Basics: How Ethanol Precipitation
of DNA and RNA Works. Bitesize Bio, pp.1–15.
Available at: https://bitesizebio.com/253/the-basics-
how-ethanol-precipitation-of-dna-and-rna-works/
[Accessed January 15, 2018].
Puritan, 2016. Swabbing for Trace Evidence vs. Swabbing
for Blood and Other Fluids. Available at:
https://blog.puritanmedproducts.com/swabbing-for-
trace-evidence-vs-swabbing-for-fluids [Accessed
January 11, 2018].
Raymond, J.J. et al., 2008. Trace DNA analysis: Do you
know what your neighbour is doing?. A multi-
jurisdictional survey. Forensic Science International:
Genetics, 2(1), pp.19–28.
Slrichantrawonq, K., Rerkamnuaychoke, B. &
Ramathibodi, M., Dna retrieval on flip-flops. , pp.30–
36.
Zumbo, P., 2013. Ethanol Precipitation. Weill Cornell
Medical College, 1932(Pauling), pp.1–12.
316
