
Magnéli-phase Ti
4
O
7
Conductive Membrane for Effective
Electrochemical Degradation of 4-chlorophenol in the
Presence of Sulfate
Y Tan and S J You
*
State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of
Technology, Harbin 150090, P. R. China
Corresponding author and e-mail: S J You, sjyou@hit.edu.cn
Abstract. Electrochemical advanced oxidation has been receiving a growing attention in
wastewater treatment because of its advantages of environmental friendliness, less secondary
pollution and ease-to-handle operation. Magnéli-phase titanium oxides (Ti
n
O
2n-1
, n=4~10)
represent the most promising electrode materials, due to its high conductivity, strong
corrosion resistance and high oxygen evolution potential. In this study, we fabricated a
Magnéli-phase Ti
4
O
7
conductive membrane for electrochemical oxidation of 4-chlorophenol
pollutant. The results demonstrated that the Ti
4
O
7
electrode has lower charge transfer
resistance and solution diffusion resistance compared with carbon cloth. Based on the
optimization of key operating parameters, the optimum electrolyte concentration, current
density and membrane flux conditions are determined to be 0.03 mol/L, 5 mA/cm
2
and 0.023
m
3
/(m
2
·s), respectively. The overall removal of 4-chlorophenol could reach the level higher
than 95% under these conditions.
1. Introduction
Recently, the electrochemical advanced oxidation process (EAOP) has attracted growing attention for
treating a variety of refractory wastewater by its virtue of environmental friendliness, less secondary
pollution, high efficiency and ease-tohandle operation [1]. At present, the feasibility of EAOP has
been verified by a large number of studies that report the treatment of wastewater containing various
pollutants such as refractory carboxylic acids [2, 3], and perfluorocarboxylic acids [4]. However,
there remain several aspects of EAOP that need further efforts if the engineered applications are to be
better implemented and developed. The most important factor that affects the performance of EAOP
is the choice of electrode materials on the basis of the electrical conductivity, electrochemical activity,
chemical stability, economic reliability and environmental friendliness. For example, the SnO
2
-doped
electrode is a high-performance electrode, but its engineered application may be limited by its
instability [5]. Likewise, the PbO
2
-doped electrode has also been used with very limited success due
to the potential of leaching of toxic lead ions into solution at anodic polarization condition [6, 7].
Although boron-doping diamond (BDD) may be expected to address these problems, its extremely
high cost and complicated fabrication are also concern in practical applications for wastewater
treatment [8, 9].
Tan, Y. and You, S.
Magnéli-phase Ti4O7 Conductive Membrane for Effective Electrochemical Degradation of 4-chlorophenol in the Presence of Sulfate.
In Proceedings of the International Workshop on Environmental Management, Science and Engineering (IWEMSE 2018), pages 437-443
ISBN: 978-989-758-344-5
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
437

Recently, it has been found that TiO
2
after establishing oxygen deficiencies within the crystalline
lattice can produce a kind of unique structure leading to a combination of excellent electrical
conductivity approaching to that of metals and great corrosion resistance close to that of ceramic
materials [10]. This material has been called Magnéli-phase titanium oxides with a generic formula
of Ti
n
O
2n-1
(n=4~10). Ti
4
O
7
offers higher oxygen evolution potential (+2.6 V vs standard hydrogen
electrode) than BDD [11]. Additionally, the Magnéli-phase titanium oxides are also cost effective
because it is made of TiO
2
, one of the most available metal oxides on the earth. These outstanding
properties make the Magnéli-phase titanium oxides particularly suitable serving as anode material for
EAOP [12].
Since the electrochemical reaction occurs on the surface of electrode, the convection of electrolyte
and diffusion of reactants cause the flow of reaction region and the uneven distribution of
concentration [13]. Based on the consideration of combining electrochemical oxidation and
membrane filtration, the electrolyte can be forced to flow inside the porous structure of conductive
membrane electrode. It will be highly desirable to increase the contact probability between
electrochemical active sites and organic pollutants, such that the mass transfer can be enhanced for
electrochemical reactions [14-17].
Herein, we have fabricated Magnéli-phase Ti
4
O
7
conductive membrane as anode for
electrochemical oxidation of 4-chlorophenol, a kind of recalcitrant contaminant that often occurs in
many industries. Following the characterization of morphology, crystal, and surface area, the
electrochemical properties of Ti
4
O
7
electrode were investigated. Thereafter, the electrochemical
oxidation performances of Magnéli-phase Ti
4
O
7
conductive membrane was examined and assessed
for removing 4-chlorophenol in synthetic wastewater. Last, the operating conditions were also
optimized and discussed.
2. Materials and methods
2.1. Characterization of the Magnéli-phase Ti
4
O
7
conductive membrane
The surface structures of the Magnéli-phase Ti
4
O
7
electrode was observed by using field-emission
scanning electron microscopy (SEM, Helios Nano-lab600i, FEI, U.S.). The powder X-ray diffraction
(XRD) analysis was conducted on an X-ray diffractometer (Bruke D8 Adv., Germany). The electrode
surface area was calculated based on the adsorption and desorption branches measured by BET
surface area measurement (3H-2000BET-A, China).
The electrochemistry impedance spectroscopy (EIS) analysis of the magnéli-phase Ti
4
O
7
electrode were tested by making a comparison with carbon cloth upon PARSTAT electrochemical
workstation (CHI 750D, Chenhua Co. Ltd., China). The EIS data obtained from the test were fitted
by using Zsimpwin software to determine the ohmic resistance, charge transfer resistance and
diffusion resistance of the tested electrode materials.
2.2. Experimental setups
The EAOP containing Magnéli-phase Ti
4
O
7
conductive membrane anode is schematically illustrated
in Figure 1. Unless stated otherwise, the experiments were conducted with 4-chlorophenol and
Na
2
SO
4
electrolyte solution. The initial concentration of 4-chlorophenol was 20 mg/L and the volume
was 580 mL. The effects of Na
2
SO
4
concentration, current density, and membrane flux on the
degradation of 4-chlorophenol were studied and optimized at the reaction time of 2 h.
2.3. Analyses methods
The samples were taken of 1.00 mL at regular time intervals. The 4-chlorophenol was determined by
using High Performance Liquid Chromatography (HPLC, Waters 2695, U.S.A) with a C18 column
(250×4.6, 5 μm) and a photodiode array detector (wavelength=254 nm). The flow phase was V
IWEMSE 2018 - International Workshop on Environmental Management, Science and Engineering
438

(methanol): V (hyperpure water) = 80:20, and the flow rate was 1.0 mL·Min
-1
. The sampling amount
was 20 μL and the column temperature was 25 °C .
Figure 1. The scheme of lab-scale EAOP reactor containing Magnéli-phase Ti
4
O
7
conductive
membrane for electrochemical oxidation of 4-chlorophenol.
3. Results and discussion
3.1. Electrode characterization
The XRD profiles (Figure 2A) were indexed to the characteristic peaks of Ti
4
O
7
according to
reference spectrum (JCPDS No. 18-1402) and prior literatures [18]. At the same time, a small amount
of diffraction peaks of Ti
5
O
9
was also shown in the profiles, which indicated that it is difficult to
generate a single Ti
4
O
7
phase during the preparation of the titanium oxide material. Figure 2B
provided the surface morphology structures of the magnéli-phase Ti
4
O
7
electrode, and results
indicated surficial pores on the order of 1-2 μm. The phenomenon of sintering and melting caused
particles to adhere to each other, thus forming a rich porous structure, which expected beneficial for
facilitating interfacial mass transfer during electrochemical reaction with the electrode BET surface
area of 0.1884 m
2
·g
-1
.
3.2. Electrochemical properties
The ohmic resistance of monolithic Ti
4
O
7
electrode was measured to be 58.07 Ω, which was an
indication of high conductivity similarly like the carbon cloth (Figure 2C). However, further analysis
of the EIS data revealed that the charge transfer internal resistance and diffusion internal resistance of
the two electrodes are quite different. The charge transfer internal resistances of the Ti
4
O
7
electrode
and the carbon cloth anode were 10 Ω and 588.3 Ω respectively, the former was far lower than the
latter. The diffusion internal resistance of the Ti
4
O
7
electrode was 11.89 Ω, while that of the carbon
cloth was 3411 Ω, which was two orders of magnitude higher than that of the Ti
4
O
7
electrode. It
means that under the same reaction conditions, the Ti
4
O
7
electrode is more conducive to the diffusion
and reaction of the solute in the solution during the reaction process, which provides a good
condition for the electrochemical reaction to take place quickly and efficiently.
Magnéli-phase Ti4O7 Conductive Membrane for Effective Electrochemical Degradation of 4-chlorophenol in the Presence of Sulfate
439

Figure 2. (A) XRD profiles,
(B) SEM observation of Magnéli-phase
Ti
4
O
7
conductive membrane
(C) Nyquist plot obtained from EIS
measurements.
3.3. Electrochemical oxidation of 4-chlorophenol
3.3.1. Effect of Na
2
SO
4
concentration on removal of 4-chlorophenol. At a current density of 5
mA/cm
2
, the synthetic 4-chlorophenol wastewater was electrolyzed with the Na
2
SO
4
concentration in
the electrolyte solution increased from 0.02 mol/L to 0.10 mol/L, the removal rate is shown in Figure
3A. It can be seen that the removal rate of 4-chlorophenol increases from 87.78% to 100% as the
concentration of electrolyte increases from 0.02 mol/L to 0.04 mol/L. When the electrolyte
concentration was continued to increase further, the removal rate of 4-chlorophenol started to reduce,
and at a electrolyte concentration of 0.10 mol/L, the 4-chlorophenol removal rate dropped to 43.21%.
Considering multiple factors comprehensively, 0.03 mol/L was used as the optimal sodium
electrolyte concentration condition in this paper.
3.3.2. Effect of current density on the removal of 4-chlorophenol. At a concentration of sodium
sulfate in the electrolyte solution of 0.03 mol/L, the synthetic 4-chlorophenol wastewater was
electrolyzed with the current density increased from 2.5 mA/cm
2
to 10 mA/cm
2
, the removal rate is
shown in Figure 3B. Within the experiment time range of 120 min, the removal rates of 4-
chlorophenol were 78.14%, 95.17%, 96.78% and 97.54%, respectively. From the data, it can be seen
that the removal rate of 4-chlorophenol increases with the current density, but the increasing rate
gradually decreases. When the current density increases from 2.5 mA/cm
2
to 10 mA/cm
2
in turn, the
removal rate increased by 21.79%, 1.69%, 0.79%. It means the percentage increase of 4-
chlorophenol removal rate is far less than that of current density and electrolysis under large current
IWEMSE 2018 - International Workshop on Environmental Management, Science and Engineering
440
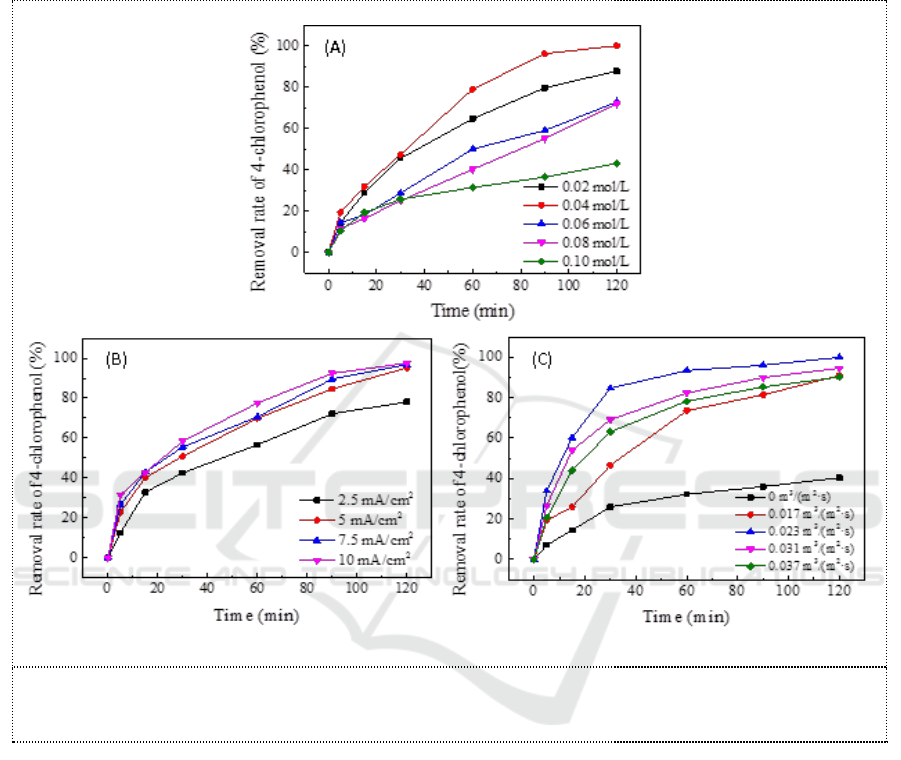
density obviously causes great waste of electric energy. Therefore, in the electrolysis reaction, the
current density should be appropriately increased within a certain range, which cannot be too large to
cause the waste of energy. This study selected 5 mA/cm
2
as the optimal current density of the
experiment.
Figure 3. Effects of (A) Na
2
SO
4
electrolyte concentration, current density (B) and membrane
flux (C) on removal of 4-chlorophenol.
3.3.3. Effect of membrane flux on the removal of 4-chlorophenol. When the concentration of the
electrolyte solution was 0.03 mol/L and the current density was 5 mA/cm
2
, the membrane flux was
adjusted by changing the rotation speed of the peristaltic pump. 0, 0.017 m
3
/(m
2
·s), 0.023 m
3
/(m
2
·s),
0.031 m
3
/(m
2
·s), 0.037 m
3
/(m
2
·s) were selected as flux gradients to apply to the Magnéli-phase Ti
4
O
7
electrochemical membrane, the removal rate is shown in Figure 3C. At 0 m
3
/(m
2
·s), there was no
suction of the peristaltic pump and only the Magnéli-phase Ti
4
O
7
electrode played the role of
electrolysis. After 60 min, the removal rate of 4-chlorophenol reached 32.32%, and then the removal
process was almost performed stagnated. Compared with zero flux, i.e. 0 m
3
/(m
2
·s), the removal rate
of 4-chlorophenol increased significantly after increasing the suction of peristaltic pump, and could
reach more than 90% under all membrane fluxes. However, it was worth noting that the removal rate
of 4-chlorophenol did not increase with the increase of membrane flux, but first increased and then
decreased. At the end of the experiment, the removal rate was 90.98%, 100%, 94.59% and 90.21%,
respectively. Taken together, 0.023 m
3
/(m
2
·s) was selected as the optimal membrane flux condition
in this study.
Magnéli-phase Ti4O7 Conductive Membrane for Effective Electrochemical Degradation of 4-chlorophenol in the Presence of Sulfate
441

4. Conclusions
In this study, the Magnéli-phase Ti
4
O
7
conductive membrane was fabricated for electrochemical
oxidation of 4-chlorophenol in the presence of sulfate electrolyte. The Ti
4
O
7
electrode achieved
efficient abatement of recalcitrant organic pollutants without any extra addition of chemicals.
The XRD profiles were indexed to the characteristic peaks of Ti
4
O
7
and a small amount of
diffraction peaks of Ti
5
O
9
for the membrane electrode. The Ti
4
O
7
electrode had surficial pores on the
order of 1-2 μm and BET surface area of 0.1884 m
2
·g
-1
, which would be beneficial for facilitating
interfacial mass transfer.
The Ti
4
O
7
electrode has lower charge transfer resistance and solution diffusion resistance
compared with the carbon cloth, suggesting high activity for oxygen evolution to produce hydroxyl
radicals and organic degradation at anodic polarization conditions.
Based on the optimization of key operating parameters, the optimum Na
2
SO
4
electrolyte
concentration, current density and membrane flux conditions were determined to be 0.03 mol/L, 5
mA/cm
2
and 0.023 m
3
/(m
2
·s), respectively. More than 95% 4-chlorophenol could be removed under
these conditions.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (No. 51678184) and
the State Key Laboratory of Urban Water Resource and Environment (Grant No. 2015TS01).
References
[1] Martínezhuitle C A, Rodrigo M A, Sirés I and Scialdone O 2015 Single and coupled
electrochemical processes and reactors for the abatement of organic water pollutants: a
critical review Chemical Reviews 115(24) pp 13362-13407
[2] Garciasegura S and Brillas E 2011 Mineralization of the recalcitrant oxalic and oxamic acids
by electrochemical advanced oxidation processes using a boron-doped diamond anode
Water Research 45(9) pp 2975-2984
[3] Queiroz J L A D, Silva A R L D, Moura D C D, Silva D R D and Martínez-Huitle C A 2017
Electrochemical study of carboxylic acids with nb-supported boron doped diamond anode.
part 1: potentiodynamic measurements and bulk oxidations Journal of Electroanalytical
Chemistry 794 pp 204-211
[4] Gomez-Ruiz B, Gómez-Lavín S, Diban N, Boiteux V, Colin A, Dauchy X and Urtiaga A 2017
Boron doped diamond electrooxidation of 6:2 fluorotelomers and perfluorocarboxylic acids.
Application to industrial wastewaters treatment Journal of Electroanalytical Chemistry 798
pp 51-57
[5] Liu H, Vajpayee A and Vecitis C D 2013 Bismuth-doped tin oxide-coated carbon nanotube
network: improved anode stability and efficiency for flow-through organic electrooxidation
Acs Applied Materials & Interfaces 5(20) pp 10054-10066
[6] Li X, Pletcher D and F Walsh C 2011 Electrodeposited lead dioxide coatings Chemical Society
Reviews 40(7) p 3879
[7] Vargas R, Borrás C, Méndez D, Mostany J and Scharifker B R 2016 Electrochemical oxygen
transfer reactions: electrode materials, surface processes, kinetic models, linear free energy
correlations, and perspectives Journal of Solid State Electrochemistry 20(4) pp 875-893
[8] Shestakova M and Sillanpää M 2017 Electrode materials used for electrochemical oxidation of
organic compounds in wastewater Reviews in Environmental Science & Bio/technology
16(2) pp 223-238
[9] Yu X, Zhou M, Hu Y, Serrano K G and Yu F 2014 Recent updates on electrochemical
degradation of bio-refractory organic pollutants using BDD anode: a mini review
Environmental Science & Pollution Research 21(14) pp 8417-8431
IWEMSE 2018 - International Workshop on Environmental Management, Science and Engineering
442

[10] Padilha A C M, Osorio-Guillén J M, Rocha A R and Dalpian G M 2014 Ti
n
O
2n-1
, Magnéli
phases studied using density functional theory Phys.rev.b 90(3)
[11] You S, Liu B, Gao Y, Wang Y, Tang C Y, Huang Y and Ren N 2016 Monolithic Porous
Magnéli-phase Ti
4
O
7
, for Electro-oxidation Treatment of Industrial Wastewater
Electrochimica Acta, 214 pp 326-335
[12] Liang S, Lin H, Yan X and Huang Q 2018 Electro-oxidation of tetracycline by a Magnéli
phase Ti
4
O
7
, porous anode: kinetics, products, and toxicity Chemical Engineering Journal
[13] Su J 2011 Characterization of fluid dynamics and mass transfer in an Electrochemical
Oxidation Reactor for organic pollutants (China: Jilin University)
[14] Zaky A M and Chaplin B P 2014 Mechanism of p-Substituted Phenol Oxidation at a Ti
4
O
7
Reactive Electrochemical Membrane Environmental Science & Technology 48(10) pp
5857-5867
[15] Zaky A M and Chaplin B P 2013 Porous Substoichiometric TiO
2
Anodes as Reactive
Electrochemical Membranes for Water Treatment Environmental Science & Technology
47(12) p 6554
[16] Guo L, Jing Y and Chaplin B P 2016 Development and Characterization of Ultrafiltration TiO
2
Magnéli Phase Reactive Electrochemical Membranes Environmental Science & Technology
50(3) pp 1428
[17] Guo L, Ding K, Rockne K, Duran M and Chaplin B P 2016 Bacteria inactivation at a sub-
stoichiometric titanium dioxide reactive electrochemical membrane Journal of Hazardous
Materials 319 pp 137-146
[18] Regonini D, Dent A C E, Bowen C R, Pennock S R and Taylor J 2011 Impedance
spectroscopy analysis of Ti
n
O
2n-1
Magnéli phases Materials Letters 65(23) pp 3590-3592
Magnéli-phase Ti4O7 Conductive Membrane for Effective Electrochemical Degradation of 4-chlorophenol in the Presence of Sulfate
443
