
Simultaneous H
2
Production with Carbon Storage by Enhanced
Olivine Weathering in Laboratory-scale: An Investigation of CO
2
Effect
Jiajie Wang
*
, Kengo Nakamura, Noriaki Watanabe, Atsushi Okamoto and Takeshi Komai
Graduate School of Environmental Studies, Tohoku University, Aramaki, Aoba-Ku, Sendai, Japan
Keywords: H
2
production, CO
2
storage, Rock-water reaction, Olivine, CO
2
-rich.
Abstract: Hydration of olivine ((Mg
,
Fe)
2
SiO
4
) potentially offers significant H
2
supply. However, because of the low
Fe(II) dissolution rate, H
2
production rate is poorly limited. In the present study, to investigate the CO
2
effect
on H
2
generation and minerals evolution, CO
2
-rich (0.5 mol/L NaHCO
3
) reaction condition was created in the
ongoing olivine hydration experiment. H
2
production was continuing with a slight increasing rate after CO
2
addition. Results indicate CO
2
-rich hydrothermal reaction condition (300
o
C, 10 MPa) promoted both olivine
and brucite (Mg,Fe(OH)
2
) dissolution, which led to additional Fe(II) releasing, and consequent H
2
generation.
CO
2
was simultaneous hydrogenated to formic acid (HCOOH) by generated H
2
and carbonated to magnesite
(MgCO
3
). 0.52 mol of CO
2
was trapped in per kg of olivine in 72 h. This study suggests simultaneous multiple
energy productions and CO
2
storage can be realized by olivine weathering process when using a CO
2
-rich
hydrothermal condition.
1 INTRODUCTION
In 2016, the atmospheric carbon dioxide (CO
2
) is
403.3 ppm, having increased by 45% compared with
the pre-industrial level of 280 ppm and still increasing
at a rate of 2 ppm per year (NOAA, 2016). Among
which, up to 65% were attributed to fossil fuel
combustion (Kularatne et al., 2018). Control of global
warming and the exploration of CO
2
-free energy
sources are the main challenges in the 21st century.
Hydrogen (H
2
) is a clean CO
2
-free energy carrier.
However, most current H
2
production processes (e.g.,
steam reforming of CH
4
) need high temperature,
which was supported by the combustion of fossil
fuels. Also, this process generates CO
2
as a reaction
product (Malvoisin et al., 2013).
In recent years, H
2
was discovered from a variety
of geologic fluids. It is commonly produced during
the hydration of rocks, owing to the oxidation of
reduced Fe present in the mineral (Kelley, 1996;
Lollar et al., 2008) at hydrothermal conditions.
Olivine ((Mg,Fe)
2
SiO
4
) is the predominant mineral of
ultramafic rock, the hydration of olivine (also called
serpentinization) potentially offers significant H
2
supply. However, the rate of H
2
generation from
olivine hydration is poorly limited. The low H
2
generation rate is partially attributed to the low
olivine dissolution rate, which can be enhanced by
varying reaction conditions, such as elevating
reaction temperature. Research reported the optimum
temperature H
2
production from olivine hydration
was 300-400
o
C (Berndt et al., 1996; McCollom and
Seewald, 2001). On the other hand, dissolved Fe(II)
prone to be incorporated into brucite (Mg,Fe(OH)
2
),
the secondary mineral of olivine hydration. This
process decreases dissolved Fe(II) in the fluid, thus
fewer Fe(II) can be oxidized, which severely
suppresses H
2
yield (Klein et al., 2009).
The presence of bicarbonates (HCO
3
-
) can
promote olivine and brucite dissolution (Matter and
Kelemen, 2009; Gerdemann et al., 2007; Harrison et
al., 2013). High concentrations of HCO
3
-
in solution
plays as a buffer to maintain relatively weakly
alkaline pH, which has an enhancement in olivine
dissolution. (Harrison et al., 2013) proposed HCO
3
-
can significantly promote brucite dissolution. Thus,
the CO
2
-rich condition potentially enhances Fe(II)
being dissolved, then H
2
production will be
accelerated. Although researches concerning
hydrocarbon generation based on serpentinization
process have used NaHCO
3
as the source of inorganic
carbon, the chemical reactions between HCO
3
-
and
Wang, J., Nakamura, K., Watanabe, N., Okamoto, A. and Komai, T.
Simultaneous H2 Production with Carbon Storage by Enhanced Olivine Weathering in Laboratory-scale: An Investigation of CO2 Effect.
DOI: 10.5220/0008186000830087
In The Second International Conference on Materials Chemistry and Environmental Protection (MEEP 2018), pages 83-87
ISBN: 978-989-758-360-5
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
83

minerals was rarely considered, partially because of
the extremely low concentration of HCO
3
-
(Berndt et
al., 1996; McCollom and Seewald, 2001).
Olivine carbonation process also occurs with the
presence of CO
2
. The CO
2
-rich condition, typically
0.64 M NaHCO
3
, favors olivine carbonation at < 200
o
C (Matter and Kelemen, 2009; Gerdemann et al.,
2007). However, previous studies mainly focus on
olivine serpentinization or carbonation separately,
because they occur at different temperature range.
Whether serpentinization and carbonation can
proceed simultaneously, and the effects of CO
2
-rich
fluid on serpentinization and H
2
production are less
clear.
This study was designed to investigate the
changes in H
2
generation, fluid chemistry and
secondary minerals generations after CO
2
-rich fluid
addition. The role of CO
2
on olivine serpentinization
and carbonation were revealed, as well as the
reactions for dissolved carbon: hydrogenated or
mineralized. Gas, fluid and mineral samples were
withdrawn during the reaction to reveal the
mineralogical changes over time.
2 MATERIALS AND METHODS
2.1 Materials
Purchased olivine grains were chosen for
experimental investigation, which was collected from
Damaping (China). Then olivine was ball-milled
(Pulverisette 6, Fritsch) to diameter <45 μm. The
composition of the olivine was measured using
Electron probe micro-analyzer (EPMA) and was
defined as Mg
1.8
Fe
0.2
SiO
4
. The effect of CO
2
on
olivine weathering was investigated using NaHCO
3
(Kanto Chemical, Japan).
2.2 Experimental Setup and Analytical
Methods
Experiments were performed in a closed-batch
reactor made of Hastelloy-C with an internal volume
of 170 mL (Figure 1). In each experiment, 100 mL
slurry prepared with 5±0.01 g of olivine powders and
Milli-Q water was poured into the reactor. Then the
reactor was closed and purged with N
2
gas for 10
minutes to remove dissolved O
2
in the solution and
the upper headspace. Then air outlet was closed and
N
2
gas continues to be injected to reach a certain
pressure. The reactor was well sealed to increase the
temperature to 300 °C, with the final pressure reached
10 MPa. After 72 h reaction, the reactor was opened
and NaHCO
3
was added to create CO
2
-rich (0.5
mol/L NaHCO
3
) reaction condition. Then the reactor
was sealed and the original reaction conditions (300
o
C and 10 MPa) were reset. Gas, liquid and solid
samples were withdrawn along the reaction via
sampling tubes. Solid samples withdrawn at the
reaction time of 72 and 144 h were named as O72 and
O72-C72, respectively. After 144 h, experiments
were stopped by reducing the temperature of the
reactor using recirculated cooling water. The mineral
powder was filtered and dried at 50 °C for 24 h in an
oven before further analysis.
Figure 1: Schematic diagram of the experimental set-up.
Liquid samples were analyzed using ion
chromatography (IC; 761 Compact IC, Metrohm,
Switzerland) coupled with a Metrosep Organic Acids
column (Metrohm, Switzerland), and Inductively
Coupled Plasma equipped with an Atomic Emission
Spectrometry (ICP-AES). Gas species were analyzed
using gas chromatography (GC; GC-3200, GL
Science, Japan) equipped with a thermal conductivity
detector (TCD). Mineral compositions and crystalline
structures of the minerals were measured using XRD
(Multiflex, Rigaku, Japan) with Cu Kα radiation (λ=
1.54 Å) operated at 40 kV and 20 mA, and with a 2θ
step size of 0.02° from 10° to 45°. The surface
morphologies of the minerals were observed using
scanning electron microscopy (SEM; SU-8000,
Hitachi, Japan). Thermogravimetric analyses (TGA)
of the solid samples were performed using a thermos-
dilatometer (Thermo plus EVO TG 8120, Rigaku,
Japan). The temperature was increased from room
temperature to 1000 °C at a rate of 10 °C per minutes.
The H
2
O contents of brucite and serpentine
(Mg
3
Si
2
O
5
(OH)
4
), CO
2
contents of magnesite
(MgCO
3
) could be determined separately from the
weight loss observed in different temperature ranges.
Then the mass losses due to different minerals could
be identified, and the final mineral composition was
estimated.
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
84
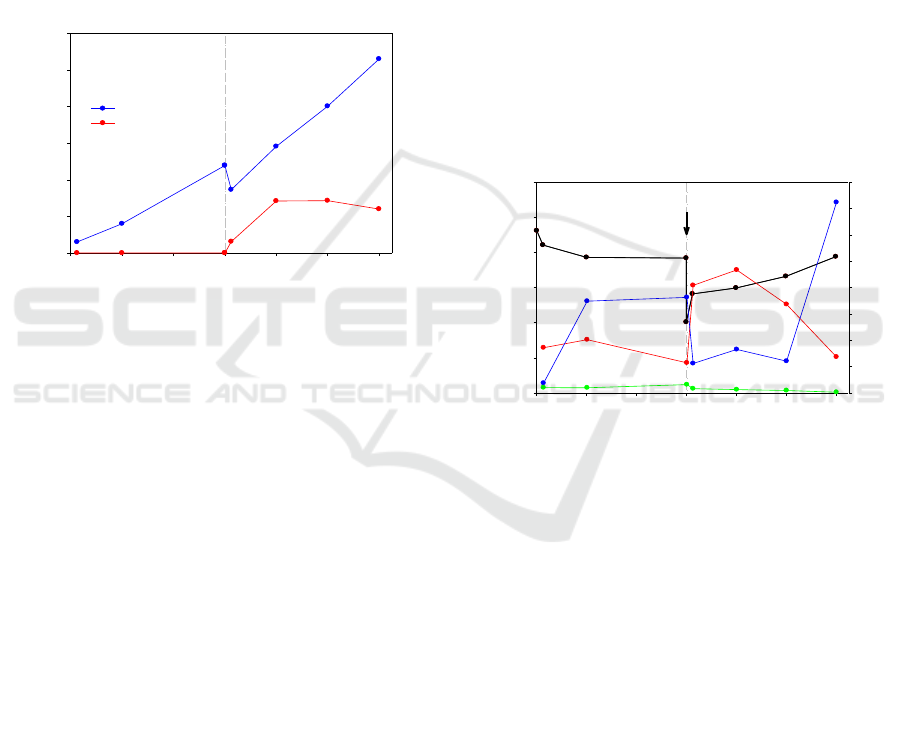
3 RESULTS AND DISCUSSION
3.1 Energy Production
During the reaction, gas and liquid samples were
withdrawn at the reaction time of 3, 24, 72, 75, 96,
120 and 144 h. Gaseous H
2
and liquid HCOOH were
experimental detected as the main products in the
present study. Products yields were measured three
times, and the average ones are summarized in Figure
2. CH
4
was also detected after 72 h reaction. However,
the concentration was too low (several μmol) to be
quantified with sufficient accuracy.
Figure 2: H
2
and HCOOH productions as function of time.
In the first 72 h, the slurry was CO
2
-free. H
2
yield
increased to 47.8 mmol/kg of olivine with olivine
hydration proceeding, suggesting that Fe(II)
dissolved from olivine was oxidized to Fe(III).
HCOOH was not detected in this stage, indicating
dissolved carbon was almost removed out from
solution via N
2
bubbling process. After 72 h reaction,
NaHCO
3
was added into the reactor to create a
concentration of 0.5 mol/L. The accumulated H
2
yield
increased to 106.0 mmol/kg of olivine after reaction
for another 72 h. The continuous generation of H
2
in
the CO
2
-rich condition indicates that Fe(II) oxidation
was not retarded. The average H
2
production rate was
increased from 0.61 mmol/kg·h before NaHCO
3
addition to 1.03 mmol/kg·h after NaHCO
3
addition.
(Klein and McCollom, 2013) reported the CO
2
addition would severely retarded H
2
production from
olivine because of dissolved Fe(II) was easier to be
incorporated into magnesite rather than be oxidized.
Compares it with the present study, the reaction
temperature may play an important role on judging
the CO
2
effect on H
2
production. Their reaction
temperature is 230
o
C while that of the present study
is 300
o
C. HCOOH was generated in the first 3h after
NaHCO
3
addition and reached stable at 28.4 mmol/kg
of olivine after 24 h. CH
4
concentration did not
increase even at the CO
2
-
rich condition with higher
HCOOH and H
2
concentrations.
3.2 Fluid Chemistry
Fluid compositions of the samples withdrawn during
the reaction are summarized in Figure 3. During the
first 72 h reaction in carbon-free condition, pH of the
slurry decreased from initial 10.62 to 9.84. Dissolved
SiO
2(aq)
concentration increased from initial 0.34 to
0.40 mol/L in the first 24 h, then followed by a slight
decrease to 0.23 mmol/L. Mg
2+
concentration
increased from initial value 0.08 to a plateau of 0.73
mmol/L, whereas Ca
2+
concentration increased
slightly from initial value 0.04 to 0.06 mmol/L. The
much smaller increase in Ca
2+
concentration is
attributed to the low Ca composition in initial olivine
particles. As the reaction progress, which was
attributed to olivine dissolution.
Figure 3: Fluid composition as a function of time.
The addition of NaHCO
3
caused rapid changes in
the fluid chemistry. Solution pH decreased from 9.84
to 8.02 immediately because of the buffering of
NaHCO
3
. In the subsequent reaction, the pH
increased to 9.49 gradually with the reaction time
reaching 144 h. The increase of pH was partially
attributed to the dissolution of olivine particles. With
the addition of NaHCO
3
, SiO
2(aq)
concentration
increased significantly from 0.23 to 0.82 mmol/L in 3
h, which indicates the enhancement on olivine
dissolution (Klein and McCollom, 2013; Gadikota et
al., 2014). As the reaction process, SiO
2(aq)
concentration eventually decreased again to 0.27
mmol/L. Mg
2+
concentration decreased immediately
from 0.73 to 0.22 mmol/L, whereas Ca
2+
concentration decreased from 0.06 to 0.03 in 3 h after
the addition of NaHCO
3
. It indicates the carbonation
process conducted quickly after CO
2
was added. At
the last 24 h of the reaction, the Mg
2+
concentration
carbon-free
reaction time (h)
0 24 48 72 96 120 144
H
2
and HCOOH yield (mmol/kg of olivine)
0
20
40
60
80
100
120
H
2
HCOOH
carbon-rich
(0) (24)
(48) (72)
0 24 48 72 96 120 144
pH
6
7
8
9
10
11
12
Si (mM)
,
Mg(mM),
Ca (mM)
0.0
.2
.4
.6
.8
1.0
1.2
1.4
1.6
carbon-free
carbon-rich
Addition of NaHCO
3
(0.5 M)
reaction time (h)
Simultaneous H2 Production with Carbon Storage by Enhanced Olivine Weathering in Laboratory-scale: An Investigation of CO2 Effect
85
increased sharply to 1.45 mmol/L, infers the retarded
carbonation process.
3.3 Mineral Changes
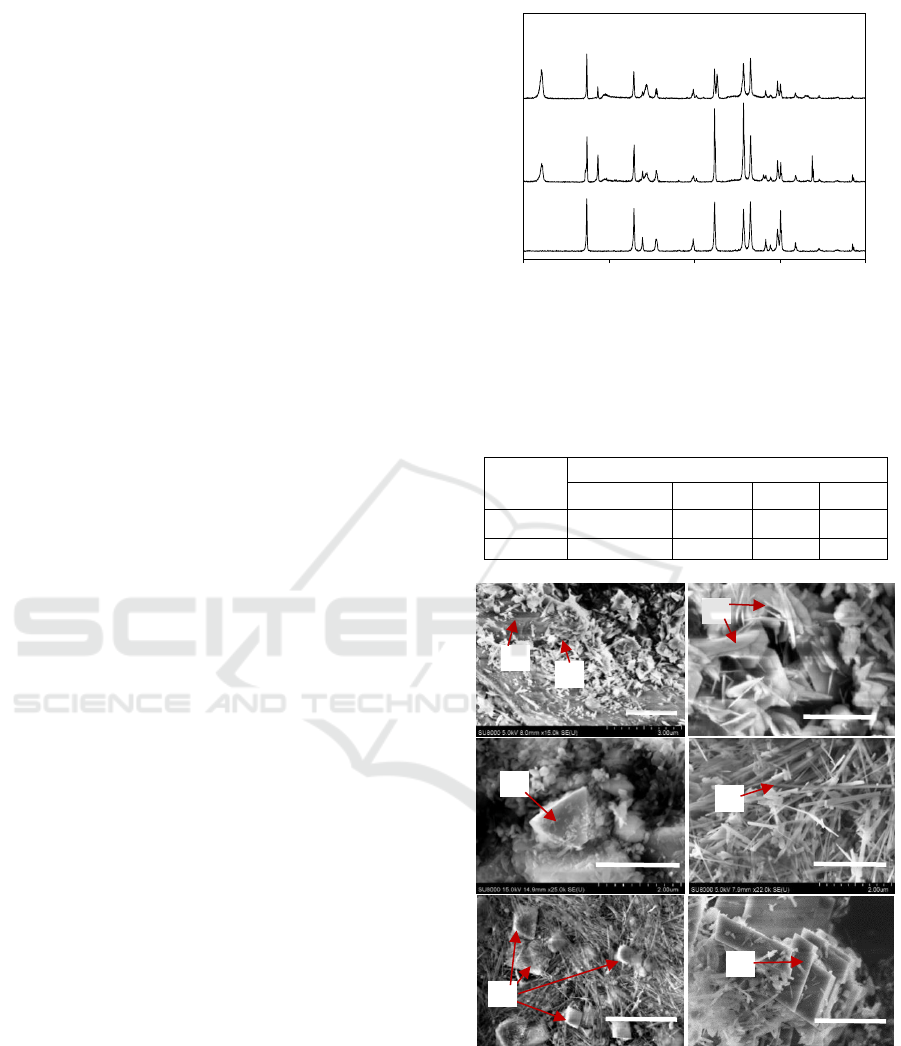
Solid after reactions were analysed using XRD to
identify mineral compositions. The results are shown
in Figure 4. The main mineral products before carbon
injection are serpentine, brucite and magnetite
(Fe
3
O
4
). Evident serpentine peaks were observed at
12.2°, 19.4° and 24.5° after 72 h reaction in CO
2
-free
condition. It infers serpentinization was the main
process for olivine weathering at this stage. Peaks at
18.8
o
and 38.1
o
are referred to brucite (Schaef et al.,
2011). Magnetite peak was found at 30.2
o
. No
magnesite peak was detected. After creating a CO
2
-
rich condition in the reactor and continuing the
reaction for another 72 h, stronger serpentine peaks
were observed from the solid sample. It indicates the
promoted serpentine generation. The peak occurred
around 32.8
o
belongs to magnesite (Rahmani et al.,
2016), which indicates the carbonation process. In
addition, brucite content was severely decreased with
much smaller brucite peaks be observed.
SEM imaging has revealed a clear evolution of
morphology of crystal face during olivine weathering
process. The dominant serpentine polymorph found is
chrysotile. Before the CO
2
addition, chrysotile fibers
have a diameter of 10-100 nm and are less than 1 μm
in length (Figure 5(a)). Brucite and few magnetites
were generated with several μm in size (Figure 5(b,
c)). After reacting in CO
2
-rich condition for another
72 h, serpentine fibrous became longer with more
than 2 μm in length (Figure 5(d)). Brucite was hardly
observed. Brucite potentially incorporates Fe(II). The
addition of NaHCO
3
in present study promoted
Fe(II)-bearing brucite dissolution, thus enhanced
Fe(II) being released, which potentially accelerated
H
2
generation. More magnetite particles were
observed according to SEM imaging (Figure 5(e)).
However, the changes in mineral yield cannot be
determined, since the observed area using SEM
observations is very limited. Magnetite is the
secondary mineral contains Fe(III), the formation of
magnetite infers the oxidation of Fe(II). In addition,
magnetite is a well-studied catalyst for CO
2
reduction, which particular catalyzes CH
4
generation.
However, in the present study, CH
4
yield was low, the
reason still needs to be explored. Magnesite, the main
products of CO
2
mineralization, was generated after
NaHCO
3
addition, as to be shown in Figure 5(f) with
rhomb shape.
Figure 4: XRD patterns of olivine, olivine after 72 h
reaction in CO
2
-free condition (O72) and after an additional
72 h reaction in CO
2
-rich condition (O72-C72). O: olivine,
S: serpentine, B: brucite, M: magnesite, Mt: magnetite.
Table 1: TGA results of mineral compositions.
Sample
Product amount (wt.%) from TGA
Serpentine
Magnesite
Brucite
Olivine
O72
12.65
0.00
0.45
86.76
O72-C72
31.05
4.07
0.00
64.33
Figure 5: SEM images of minerals after reacting for 72 h (a,
b, c) and 144 h (d, e, f). O: olivine, S: serpentine, B: brucite,
Mt: magnetite, M: magnesite.
To quantify the mineral compositions after
reactions, TGA was used. The calculated results are
shown in Table 1. The weight percent of serpentine
generated in the CO
2
-free condition in 72 h is 12.65
wt.%. After reacting in CO
2
-rich condition for
2 theta (
o
)
10 20 30 40 50
Intensity (a.u.)
O72
O
O
S
O
B
M
O72-C72
S
O
B
O
O
O
S
O
O
O
S
O
Mt
O
Mt
O
O
B
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
Olivine
O
(a)
(c)
(d)
(e)
2 μm
2 µm
5 μm
5 μm
O
B
Mt
M
S
(b)
(f)
Mt
S
2 μm
2 μm
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
86

another 72 h, serpentine composition increased by
18.40 wt.%. It indicates serpentinization process was
slightly promoted in the presence of CO
2
. Magnesite
was generated in the second stage of the experiment
with CO
2
. The yield was 4.07 wt.% of solid collected
after 72 h reaction, equivalent to trapping of 0.52 mol
of CO
2
per kg of olivine. Brucite was consumed after
CO
2
addition as no weight loss belongs to brucite was
detected in the O72-C72 solid sample.
4 CONCLUSIONS
The present study traced the changes in H
2
yield, fluid
chemistry and minerals after CO
2
addition as a
function of time. H
2
generation was continuing at the
CO
2
-free and CO
2
-rich condition. The production rate
was increased slightly after the addition of NaHCO
3
.
Olivine and brucite dissolution were accelerated in
CO
2
-rich condition, which may be attributed to pH
decrease caused by NaHCO
3
addition. The
dissolution of Fe(II)-contained brucite contributed
Fe(II) releasing, thus promoted H
2
production. Our
experiment results suggest simultaneous energy
production and CO
2
storage can be realized when
using CO
2
-rich hydrothermal condition in olivine
weathering process.
ACKNOWLEDGEMENTS
The authors thank Kawabe Yoshishige in AIST
(Japan) for helping ICP-AES analysis. The authors
also thank reviewers who gave helpful suggestions.
This work was supported by JSPS KAKENHI Grant
Number JP18J12695.
REFERENCES
Berndt, M., Allen, D. and Seyfried, W., 1996. Reduction of
CO
2
during serpentinization of olivine at 300 °C and
500 bar. Geology, 24(4), pp.351-354.
Gadikota, G., Matter, J., Kelemen, P. and Park, A., 2014.
Chemical and morphological changes during olivine
carbonation for CO
2
storage in the presence of NaCl
and NaHCO
3
. Physical Chemistry Chemical Physics,
16(10), pp.4679.
Gerdemann, S., O'Connor, W., Dahlin, D., Penner, L. and
Rush, H., 2007. Ex Situ Aqueous Mineral Carbonation.
Environmental Science & Technology, 41(7), pp. 2587-
2593.
Harrison, A., Power, I. and Dipple, G., 2012. Accelerated
Carbonation of Brucite in Mine Tailings for Carbon
Sequestration. Environmental Science & Technology,
47(1), pp. 126-134.
Kelley, D., 1996. Methane-rich fluids in the oceanic crust.
Journal of Geophysical Research: Solid Earth,
101(B2), pp. 2943-2962.
Klein, F. and McCollom, T., 2013. From serpentinization
to carbonation: New insights from a CO
2
injection
experiment. Earth and Planetary Science Letters, 379,
pp. 137-145.
Klein, F., Bach, W., Jöns, N., McCollom, T., Moskowitz, B.
and Berquó, T., 2009. Iron partitioning and hydrogen
generation during serpentinization of abyssal
peridotites from 15
o
N on the Mid-Atlantic Ridge.
Geochimica et Cosmochimica Acta, 73, pp. 6868-6893.
Kularatne, K., Sissmann, O., Kohler, E., Chardin, M.,
Noirez, S. and Martinez, I., 2018. Simultaneous ex-situ
CO
2
mineral sequestration and hydrogen production
from olivine-bearing mine tailings. Applied
Geochemistry, 95, pp. 195-205.
Lollar, B., Lacrampe-Couloume, G., Voglesonger, K.,
Onstott, T., Pratt, L. and Slater, G., 2008. Isotopic
signatures of CH
4
and higher hydrocarbon gases from
Precambrian Shield sites: A model for abiogenic
polymerization of hydrocarbons. Geochimica et
Cosmochimica Acta, 72(19), pp. 4778-4795.
Malvoisin, B., Brunet, F., Carlut, J., Montes-Hernandez, G.,
Findling, N., Lanson, M., Vidal, O., Bottero, J. and
Goffé, B., 2013. High-purity hydrogen gas from the
reaction between BOF steel slag and water in the 473–
673 K range. International Journal of Hydrogen
Energy, 38(18), pp. 7382-7393.
Matter, J. and Kelemen, P., 2009. Permanent storage of
carbon dioxide in geological reservoirs by mineral
carbonation. Nature Geoscience, 2(12), pp. 837-841.
McCollom, T. and Seewald, J., 2001. A reassessment of the
potential for reduction of dissolved CO
2
to
hydrocarbons during serpentinization of olivine.
Geochimica et Cosmochimica Acta, 65(21), pp. 3769-
3778.
NOAA, 2016. Trends in Atmospheric Carbon Dioxide.
Rahmani, O., Highfield, J., Junin, R., Tyrer, M. and Pour,
A.B., 2016. Experimental Investigation and Simplistic
Geochemical Modeling of CO
2
Mineral Carbonation
Using the Mount Tawai Peridotite. Molecules, 21(353).
Schaef, H.T., Windisch Jr., C.F., McGrail, B.P., Martin,
P.F., and Rosso, K.M., 2011. Brucite [Mg(OH
2
)]
carbonation in wet supercritical CO
2
: An in situ high
pressure X-ray diffraction study. Geochimica et
Cosmochimica Acta, 75, pp. 7458-7471.
Simultaneous H2 Production with Carbon Storage by Enhanced Olivine Weathering in Laboratory-scale: An Investigation of CO2 Effect
87
