
Adsorption of Cd (II) from Aqueous Solutions by a Hydroxyapatite-
Biochar Composite
Xiaowen Fu
1
, Lei Ji
1
, Qiang Zhang
1*
, Tianyuan Li
1
, Liwen Zheng
1
, Jianing Wang
1
and Shuhai Guo
1,2
1
Ecology Institute, Qilu University of Technology (Shandong Academy of Sciences), East Jingshi Road, Jinan, China
2
Institute of Applied Ecology, Chinese Academy of Sciences, Wenhua Road, Shenyang, China
Keywords: Biochar, Hydroxyaptite, Adsorption, Cadmium
Abstract: Based on the rice husk biochar(BC), a creative hydroxyapatite(HA)-biochar composite (HAC) was
fabricated in this study. SEM coupled with EDS, BET and XRD were employed to analyze the surface
features and pore structures of BC, BC+HA (the mixture of BC and HA) and HAC. The effects of solution
initial pH and the contact time between sorbents and Cd were also investigated. The results revealed that, on
the surface of HAC, HA was well loaded. Though initial pH and contact time would significantly influence
the adsorbing effects of Cd by all three sorbents, HAC showed a high efficient and capacity to adsorb Cd (II)
in the aqueous solutions than simple biochar, and a low cost than adding nano-HA.
1 INTRODUCTION
Heavy metals are known as one type of the most
severe contaminants in the environment because of
their toxicity and threaten to the ecological system
and human beings (Ahmad et al., 2014). Among
numbers of heavy metals, Cadmium(Cd) receives
the most concern because of its toxic, solubility,
mobility and biological accumulation (Wang et al.,
2015). The farmland and surrounding water bodies
are vulnerable to threaten from Cd contamination,
which is enhanced by industrial sludge, wastewater
irrigation, atmospheric pollutant settlement, and
utilization of organic fertilizer and pesticides (Lin et
al., 2015). Numerious former researches have
reproted several methods to solve heavy metal
contaminations from aqueous solutions, including
ion exchange, sedimentaiton, adsorption, biological
treatment, and electrokinetic remediation (Maatar
and Boufi, 2015). Among these methods, adsorption
has received more and more attentions due to their
advantages in treating heavy metals in the
enviroment, such as high efficiency, low cost, easy
to use, selectivity and so on (Tapaswi et al., 2014).
Biochar, as a form of biomass-derived black
carbon, is a type of adsorbent with high surface area
and good cation exchange property. It is generally
pyrolyzed under anaerobic condition by raw
materials of waste biomass, including agriculture
and forest by-products (Xinde et al., 2009). The
heavy metal adsorption mechanism of biochar
includes surface adsorption, metal exchange with
cations, electrostatic interactions and so on (Lu et al.,
2012). However, the utilization of biochar is limited
to its relatively low heavy metal adsorption capacity.
Therefore, researchers have developed many
modification mathods for biochar, espetially surface
modifications by combining nanoparticles to prepare
biochar-based nanocompsites (Ying et al., 2013).
After modifications, biochar’s removal ability of
heavy metals will be remarkably improved (Zhang
and Gao, 2013).
Hydroxyapatite (HA), naturaly found in bones
and teeth of annimals, has been confirmed to have
high adsorption capacity of divalent metal ions due
to the existence of immobilizing metallic cations of
P-OH groups and Ca on its surface (Saoiabi et al.,
2016). However, nano-HA has the defect of tending
to aggregate in clumps and encapsulated in aqueous
solutions, which will enfluence its surface character
and metal removal ability. Wang et al. reported that
with a macroporous adsorbent as the carrier, the
above disadvantages would be avoided (Wang et al.,
2018). Biochar-based adsorbent composited with
HA is produced to achieve desirable adsorption
properties.
In this work, a rice-husk based hydroxyapatite-
biochar composite (HAC) was fabricated to produce
Fu, X., Ji, L., Zhang, Q., Li, T., Zheng, L., Wang, J. and Guo, S.
Adsorption of Cd (II) from Aqueous Solutions by a Hydroxyapatite-Biochar Composite.
DOI: 10.5220/0008186601230126
In The Second International Conference on Materials Chemistry and Environmental Protection (MEEP 2018), pages 123-126
ISBN: 978-989-758-360-5
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reser ved
123

a higher removing capcities of Cd(II) in aqueous
solutions. The physical-chemical characteristics of
HAC were analyzed and compared with these of
biochar pyrolized from rice husk (BC) and the
physical mixture of BC and hydroxyapatite
(BC+HA). The effects of solution initial pH and
contact time were also studied.
2 MATIERALS AND METHODS
All chemical reagents used were in analytical
reagent grade and all the aqueous solutions were pre
pared with deionised water (DW). The nano-particle
hydroxyapatite (HA) was purchased from the
Macklin Biochemical (Shanghai, China). The rice
husks were derived from farmlands of Ulanhot,
Inner Mongolia, China.
2.1 Sorbent Preparation
Rice husk was applied as the raw material of biochar
in this study. The biochar was prepared as the
progress below. Rice husk was pyrolyzed in a
programmable muffle furnace under an atmosphere
of nitrogen (100 cm
3
/min). The furnace was
programmed to heat to 500°C with a rate of
10 °C/min and keep for 3 hours. Biochar (BC) was
obtained after the pyrolyzed rice husk was cooled to
room temperature naturally.
After BC was prepared, the hydroxyapatite-
biochar (HAC) composite was fabricated with the
following process. The prepared BC (10.0 g) was
first added to a H
3
PO
4
(15 M) which was stirring
vigorous to form the suspension solution A. Then a
Ca(OH)
2
(0.001 M) solution was prepared as the
solution B. The pH levels of solutions A and B were
both adjusted to about 10.0 with HCl or NaOH
(0.1M). After that, solution A and B were mixed
gradually. In the next 24 hours, the mixed solution
was stirred occasionally and the pH of which was
determined. After 24 h, the pH would achieve a
constant. Then the solution was centrifuged to
isolate the precipitate, which was washed three times
with ethanol. Finally, the HAC composite was
obtained after drying at the room temperature. The
mixture of BC and hydroxyapatite (BC+HA) was
obtained by a physical mixture and grind of BC and
the nano-particle hydroxyapatite with a ratio of 2:1.
2.2 Sorbent Characterization
The sorbents of BC, BC+HA, and HAC were all
characterized to compare similarities and differences.
Field emission scanning electron microscope
(Hitachi, SU8010) was applied to perform SEM
patterns of sorbents. The surface elemental
composition analysis of the sorbents was conducted
by energy-dispersive spectroscopy (EDS). Via a
X'Pert PRO MPD X-ray diffractometer, the XRD
patterns of sorbents were obtained. IR spectra from
4000-400 cm
−1
were measured on a Nicolet Nexus
470 FT-IR spectrometer. The specific surface area
was measured by a BET analyzer of Micromeritics
TriStar II 3flex.
2.3 Adsorption Experiments
In each adsorption kinetic experiment, 0.5 g sorbents
were added to Cd (II) solutions (500 mL, 50 mg/L)
and then stirred at 1000 rpm. Samples (0.5 mL) were
collected at noted different adsorption time (from 10
to 1500 min), filtered through the filter of 0.45 μm,
and analyzed via Atomic Absorption Spetrophoto-
meter (AAS). The impact of initial pH on adsorption
efficiency was performed by varying the solution’s
initial pH between 2 and 7 using NaOH or HNO
3
.
All solutions in experiments were performed in
40 mL brown glass vials and shaken in a
thermostatic oscillator with a speed of 150 r/min.
Through calculating the difference Cd(II)
concentrations between before and after the
adsorption equilibrium, the adsorption capacities of
adsorbents were valued. There were three replicates
for all experiments in this study. Standard solutions
were determined with every ten samples. The
recovery percentages were 97.6-104.3%, and the
relative standard deviations were below 2.45%.
3 RESULTS AND DISCUSSION
3.1 Characterization of Sorbents
The elemental contents of BC, BC+HA and HAC
are shown in Table 1, indicating significant
differences between three materials. BC+HA and
HAC both showed significant decrease in surface
carbon content and increase in surface oxygen
content compared to BC. This result implied the
existence of larger amounts of oxygen-containing
functional groups and thus higher polarity in the
sorbents of HAC and BC+HA than BC. The results
of specific surface areas (S
BET
), and average pore
width of BC, BC+HA and HAC (Table 1) revealed
that all materials showed mesoporous structures. The
significant decrease of surface area of HAC
suggested the occupation of pores of BC by HA,
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
124
which could also explained by the increase of
average pore width.
Table 1: Selected physiochemical properties of BC,
BC+HA and HAC.
Sorbents
BC
BC+HA
HAC
Surface atomic composition (%)
C
66.8±1.08
18.1±0.44
16.1±0.61
O
29.1±1.39
38.9±0.67
37.9±0.77
P
-
11.6±0.18
3.79±0.15
Ca
-
22.5±0.29
16.0±0.29
S
BET
(m
2
/g)
204.8
193.7
68.64
Pore width
(nm)
4.34
14.36
12.41
SEM images of BC, BC+HA and HAC showed
the clear differences among three sorbents. BC
showed a porous structure on its surface which
indicating adsorption sites (Figure 1a). The surface
of BC+HA and HAC both featured larger numbers
of particles (Figure 1b&c), which could be thought
as composing of hydroxyapatite deposited on the
surface of biochar. This conclusion was supported
by the fact of the increased percentages of Ca and P
on the surface of BC+HA and HAC (Table 1).
Figure 1: Electron microscopy of BC (a), BC+HA (b) and
HAC (c).
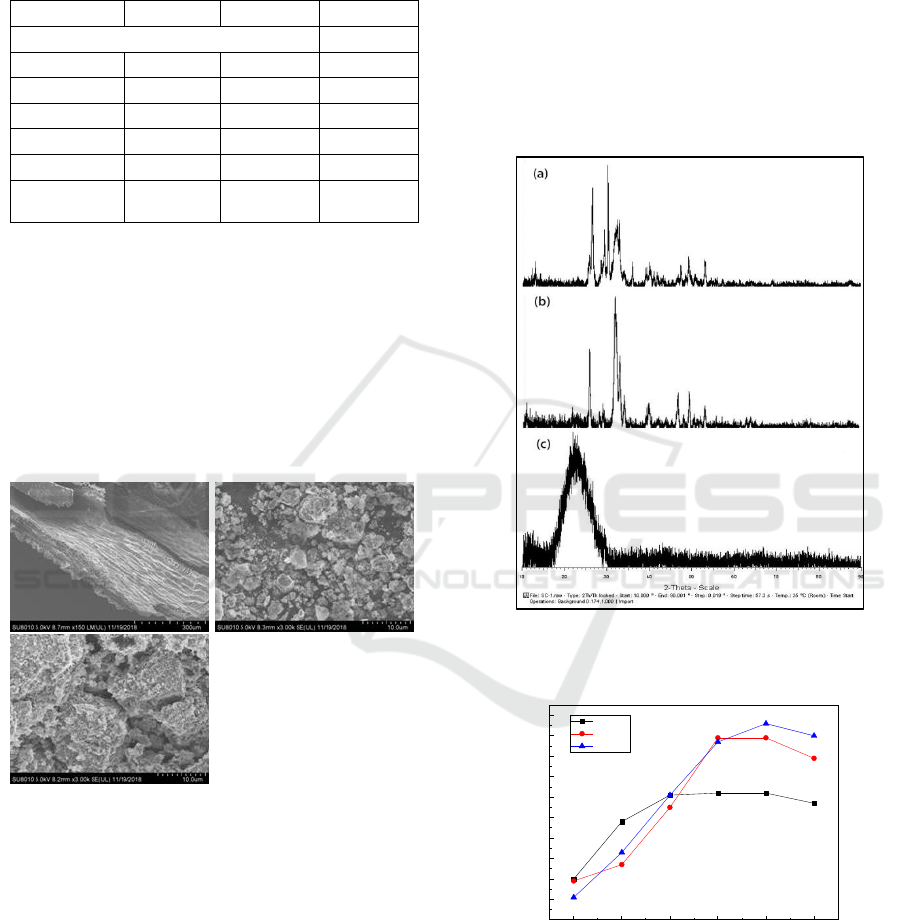
The XRD results of BC, BC+HA and HAC were
shown in Figure 2. The pattern of BC shows a broad
hump peak at 23°, which indicates that the sample
was amorphous. The XRD patterns of BC+HA and
HAC were similar and significantly different from
that of BC, indicating that HAP was loaded on the
surface of the two biochars of BC+HA and HAC.
3.2 Effects of Initial pH
Figure 3 shows that initial pH is a very vital factor
that affected the adsorption rates and processes of
BC, BC+HA and HAC. The Cd (II) adsorption
capacity increased when the initial pH ranged from 2
to 6, due to the electrostatic interactions were
facilitated by affecting the electric charge density of
the surface, resulting the increase of adsorption rate
(Jefferson et al., 2015). However, when the initial
pH was higher than 7, the metal-deposition reactions
of Cd(II) led to the decrease of soprtion rates .
Figure 2: XRD patterns of BC (c), BC+HA (a) and HAC
(b).
2 3 4 5 6 7
0
10
20
30
40
50
60
70
80
90
100
uptake percentage (%)
pH
BC
BC+HA
HAC
Figure 3: Effects of initial pH on the adsorptions of Cd.
3.3 Sorption Kinetics
Figure 4 revealed the Cd(II) sorption kinetics of
three adsorbents in aqueous solutions. The
adsorption rates increased quickly during the first
(a)
(b)
(c)
Adsorption of Cd (II) from Aqueous Solutions by a Hydroxyapatite-Biochar Composite
125

200 mins. Almost 90% of the ultimate sorption
occurred in the first 300 mins for all three sorbents,
and follwed with a quick approach to the
equilibrium. The Cd (II) removal percentages by BC,
BC+HA and HAC at equilibrium were 41.1%,
80.5%, and 82.7%, respectively. To fit the
experimental data, equations of pseudo first order
and pseudo second order were both used. For HAC,
the R
2
value of pseudo-second-order model (0.992)
was relatively higher than that of pseudo-second-
order model (0.982), indicating the chemisorption
involved between Cd(II) and sorbents in the sorption
process.
0 200 400 600 800 1000 1200 1400 1600
0
5
10
15
20
25
30
35
40
45
BC
BC+HA
HAC
pseudo-1st-order of BC
pseudo-1st-order of BC+HA
pseudo-1st-order of HAC
pseudo-2nd-order of BC
pseudo-2nd-order of BC+HA
pseudo-2nd-order of HAC
sorption amount (mg/g)
time (min)
Figure 4: Cd(II) sorption kinetics of BC, BC+HA and
HAC.
4 CONCLUSIONS
Based on the rice husk biochar and the
hydroxyapatite, a hydroxyapatite-biochar composite
was fabricated in this study. Through characteristics,
effect of initial pH and kinetic analysis, HAC
showed a better sorption performace than pure
biochar and a lower cost than physical mixture of
biochar and hydroxyapatite. The results revealed that
HAC exhibited a potential application as an
excellent sorbent for Cd (II) reduction from polluted
waters.
ACKNOWLEDGEMENTS
This research was supported by the following funds:
the National Science Foundation of China
(41807111), the Shandong Provincial Natural
Science Foundation, China (ZR2016YL002), and the
Research Project (Youth Fund) of Shandong
Academy of Sciences (2017QN007).
REFERENCES
Ahmad, M., A. U. Rajapaksha, J. E. Lim, Z. Ming, N.
Bolan, D. Mohan, M. Vithanage, S. L. Sang and S. O.
Yong, 2014. Biochar as a sorbent for contaminant
management in soil and water: A review.
Chemosphere 99(3): 19-33.
Jefferson, W. A., C. Hu, H. Liu and J. Qu, 2015. Reaction
of aqueous Cu–Citrate with MnO 2 birnessite:
Characterization of Mn dissolution, oxidation products
and surface interactions. Chemosphere 119: 1-7.
Lin, W., X. Cui, H. Cheng, C. Fei, J. Wang, X. Zhao, C.
Lin and P. Xiao, 2015. A review of soil cadmium
contamination in China including a health risk
assessment. Environmental Science & Pollution
Research 22(21): 16441-16452.
Lu, H., W. Zhang, Y. Yang, X. Huang, S. Wang and R.
Qiu, 2012. Relative distribution of Pb 2+ sorption
mechanisms by sludge-derived biochar. Water
Research 46(3): 854-862.
Maatar, W. and S. Boufi, 2015. Poly(methacylic acid-co-
maleic acid) grafted nanofibrillated cellulose as a
reusable novel heavy metal ions adsorbent.
Carbohydrate Polymers 126: 199-207.
Saoiabi, S., A. Gouza, H. Bouyarmane, A. Laghzizil and
A. Saoiabi, 2016. Organophosphonate-modified
hydroxyapatites for Zn(II) and Pb(II) adsorption in
relation of their structure and surface properties.
Journal of Environmental Chemical Engineering 4(1):
428-433.
Tapaswi, P. K., M. S. Moorthy, S. S. Park and C. S. Ha,
2014. Fast, selective adsorption of Cu 2+ from
aqueous mixed metal ions solution using 1,4,7-
triazacyclononane modified SBA-15 silica adsorbent
(SBA-TACN). Journal of Solid State Chemistry
211(5): 191-199.
Wang, H., B. Gao, S. Wang, J. Fang, Y. Xue and K. Yang,
2015. Removal of Pb(II), Cu(II), and Cd(II) from
aqueous solutions by biochar derived from KMnO 4
treated hickory wood. Bioresource Technology 197:
356-362.
Wang, Y. Y., Y. X. Liu, H. H. Lu, R. Q. Yang and S. M.
Yang, 2018. Competitive adsorption of Pb(II), Cu(II),
and Zn(II) ions onto hydroxyapatite-biochar
nanocomposite in aqueous solutions. Journal of Solid
State Chemistry 261: 53-61.
Xinde, C., M. Lena, G. Bin and H. Willie, 2009. Dairy-
manure derived biochar effectively sorbs lead and
atrazine. Environmental Science & Technology 43(9):
3285-3291.
Ying, Y., G. Bin, C. Jianjun and Y. Liuyan, 2013.
Engineered biochar reclaiming phosphate from
aqueous solutions: mechanisms and potential
application as a slow-release fertilizer. Environmental
Science & Technology 47(15): 8700-8708.
Zhang, M. and B. Gao, 2013. Removal of arsenic,
methylene blue, and phosphate by biochar/AlOOH
nanocomposite. Chemical Engineering Journal
226(24): 286-292.
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
126
