
Physico-chemical Characterization, Structuration and Morphology of
Photo-active Heterojunction (P3HT-PCBM) used in Organic
Photovoltaic Cells
S. Hamham, S. Belaaouad and Y. Naimi
Laboratory chemical chemistry of materials Hassan II University Faculty of Science Ben M'sik Department of Chemistry,
Morocco
Keywords: Photoactive Layer, Lamellar Structuration, Quasi-crystalline Fibrils, Nanocrystals PCBM, Electron Donor
Acceptor Interface.
Abstract: In this paper, we propose a study on the physicochemical, morphological and structural characterization of
the organic semiconductors (P3HT) (poly-3-hexylthiophene) and the (PC61BM) ([6,6] - phenyl - C61 -
butyric acid methyl ester), as well as their structuration in lamellae of quasi-crystalline fibrils for the first
one and in Nano-crystals for the second. These conformational transitions that the two macromolecules
undergo allow their self-assembly in chlorobenzene into a photoactive donor-electron acceptor layer. By
controlling the morphology of the layer, its film deposition on the electrodes and its orientation with respect
to which, a donor charge transfers optimization (P3HT) is favoured to the PC61BM acceptor which aims to
improve the efficiency of the organic photovoltaic cells.
1 INTRODUCTION
The study and characterization of the
physicochemical properties of semiconducting
materials based on conjugated polymers has been the
subject of numerous studies, however, we are faced
with a panoply of problems and difficulties posed by
their use which are far to be solved and continue to
receive increased attention and research (Destruel
and Seguy, 2007), (Vanlaeke et al., 2006), (Baran et
al., 2017).
The materials of choice on which we will focus
this study, will be P3HT (poly (3-hexylthiophene))
as electron donor and PC61BM ([6.6] -phenyl-C61-
butyric acid methyl ester) as electron acceptor
(Berson et al., 2007). Which will be used to develop
a one-dimensional network of organization, structure
and shape thus optimizing the charge transfer by
opting for the low oxidation potential of the donor,
which results in the reduction of the width of the
forbidden band. In addition, the presence of the
σ_π_σ bonds, which lead to the conjugation of the
chain, and constitute traps for the electrons, which
are essential to preserve the structuration and
organization of the P3HT-PC61BM, network which
is necessary for the transport of Frenkelexcitons of
small radius because of the low dielectric constant of
the two materials (Björströmet al., 2005), (Brabec et
al., 2010), (Alet et al., 2006).
Therefore, the purification and obtaining of high
molecular weight polymers and high regio-regularity
is the key to the development of the ordered
structure network and the electron-donor-acceptor
interface. Different techniques for preparing,
characterizing and depositing the active layer on the
substrate and on the electrodes in order to obtain the
volume heterojunction capable of collecting the
photons and generating the excitons, which will
dissociate into charge carriers. These processes will
provide access to pure polymers, their molecular
weight, their chemical, electrochemical, thermal,
electrical and optical properties, and finally to their
optimum size and shape ensuring their self-assembly
in order volume heterojunction providing access to
improved energy conversion efficiency of organic
photovoltaic cells (Chen et al., 2011), (Dang, Hirsch
and Wantz, 2011), (Rispenset al., 2003), (Peumans,
Yakimov and Forrest, 2003).
218
Hamham, S., Belaaouad, S. and Naimi, Y.
Physico-chemical Characterization, Structuration and Morphology of Photo-active Heterojunction (P3ht-Pcbm) Used In Organic Photovoltaic Cells.
DOI: 10.5220/0009776102180225
In Proceedings of the 1st International Conference of Computer Science and Renewable Energies (ICCSRE 2018), pages 218-225
ISBN: 978-989-758-431-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
2 SYNTHESIS AND
CHARACTERIZATION OF THE
ACTIVE LAYER
2.1 P3HT Donor Material
2.1.1 Synthesis and Spectroscopic
Characterization
The synthesis is carried out starting from a starting
material, which is 3bromo- 4-methylthiophene-C11
in two stages starting from 4 g of the reagent, an
alkylation followed by an aromatic nucleophilic
substitution in the presence of Cu (I), then the
brominated derivatives are purified by evaporation
after isolation by chlorobenzene extraction of 3
hexyloxytiophene. For the polymerization, several
processes are possible: Electrochemical
polymerization, polymerization by chemical
coupling of two aromatic rings catalyzed by
complexes with transition metals, or opt for the
polymerization by oxidizing chemical means the
presence of (FeCl3, MoCl5, RuCl3) and then
eliminates the mineral impurities methanol hot
followed by training via a cyclohexane washing and
finally proceeded to a purification by washing with
soxhlet.
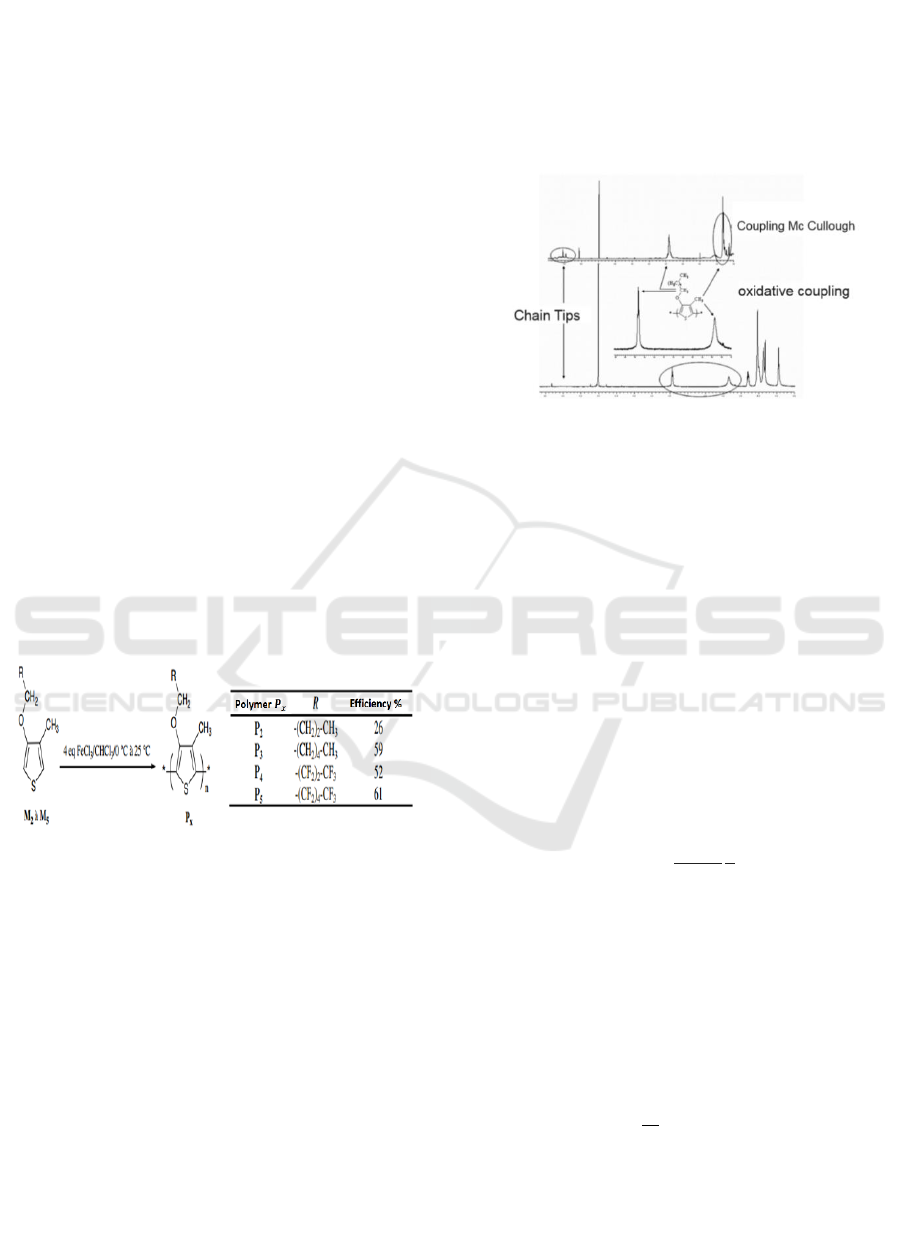
Figure 1: Synthesis scheme and polymerization yields of
P2 to P5.
After extraction of the polymers with
dichloromethane or with chloroform, the compound
is isolated by filtration-centrifugation. And in order
to control the regioregularity due to the presence of
hexyl and hexyloxyl chains which conditions the
electrical and photovoltaic properties of the P3HT
by, playing either on the temperature of the reaction
or the steric hindrance, and it is found that the longer
the polymer chain is, the higher its molecular
weight, and the greater the degree of regioregularity
which allows the polymeric chains of organize in
ordered lamellar structure of fibrils, which are
detected by UV absorption spectroscopy, and the
resistance of fibril amalgams (lamellae) which is due
to the interaction between the lateral alkyl chains is
confirmed by 1 H NMR spectroscopy, the aromatic
protons close to the thiophene ring junctions are
influenced by these and the aliphatic protons of CH3
and CH2 are influenced by the couplings, which
makes it possible to observe the existence of long
chains, thus of high molecular weight, and this gives
them a high degree of regioregularity.
Figure 2: Comparison of the proton NMR spectra in
tetrachloroethane of poly (3-hexyloxy-4-methylthiophene)
obtained by chemical coupling of Mc Cullough P3't type
by oxidative coupling P3.
2.1.2 Measurement of the Molar Mass of
P3HT
Many techniques are existing for determination
of molar mass of macromolecules in solution for
example: viscosimetry, osmometry, polyacrylamid
gel electrophoresis PAGE, static light scattering
SLS, steric exclusion chromatography SEC,
relaxation time visco-elastometry RTVE and
ultracentrifugation ...etc.
The molar mass is obtained specially by
measurements of the viscoelastic relaxation time
expressed by the Zimm-Rouse relationship:
Molar mass of the molecule
. Form factor.
T: Temperature.
R: Constant of perfect gases.
: Viscosity of the pure solvent.
Viscosity of the polymer in solution.
Intrinsic viscosity of the solution of the polymer
given by the formula of Staudinger:
Where K and α are constants and C is the
concentration of the solution.
(2)
(1)
Physico-chemical Characterization, Structuration and Morphology of Photo-active Heterojunction (P3ht-Pcbm) Used In Organic
Photovoltaic Cells
219
For de macromolecules on the long chain, we
have the semi empirical relation:
(3)
The molar mass of P3HT in solution can also be
measured by SEC molecular exclusion
chromatography using the following formula:
(4)
Or a and b are constants, K and α depend on the
geometry of the studied polymer
Volume of the elapsed buffer of the column
before the exit of the polymer studied.
Volume of the elapsed buffer of the column
before the exit of the small molecules.
: Volume of the elapsed buffer of the column
before the release of very large molecules.
It is even possible to use static light scattering
(SLS), gel electrophoresis, osmometry, translational
diffusion, ultracentrifugation (sedimentation mass
M
z
), etc., to measure the molar masses on the
number and on the weight of the polymer studied
P3HT, which have the value:
(by SEC and osmometry)
(by SLS and SEC)
And an apparent molar mass of the
macromolecule dependent on the molecular
anisotropy δ by equation:
(5)
The parameter is obtained by used of flow
birefringence
Table 1: Molecular masses and polydispersity index
obtained by SEC in solution in polystyrene equivalent
THF for the batch of P3HT from Aldrich and Rieke.
M
n
[g/mol Eq
PS]
M
w
[g/mol Eq
PS]
I
p
P3HT lot
aldrich
22 620
33 910
1.50
P3HT lot
Rieke
24 180
37 590
1.50
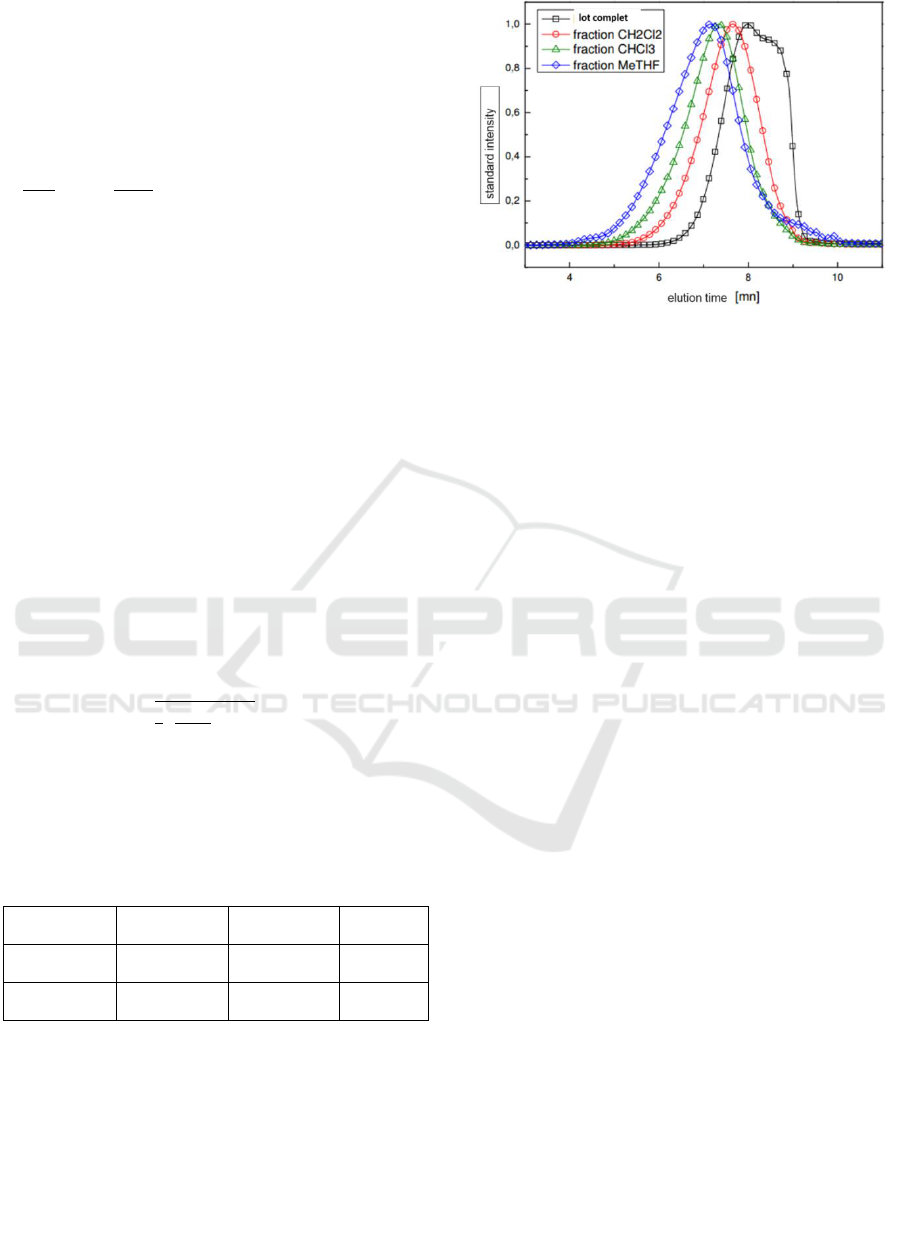
Figure 3: Chromatogram obtained for the different
fractions of P3.
This chromatogram thus reveals the presence of
three fractions obtained from the polymer P3 in
addition to the complete p3 as a function of their
elution time different can be distinguished and
separated and by an optical method
(spectrophotometry, differential refractometry, ...)
can determine their abundance and also one can
have information on the distributions of their molar
masses.
2.2 Acceptor Material PCBM
Methyl [6,6] -phenyl-C61-butanoate PCBM is an
electron-accepting organic semiconductor consisting
of a C
61
fullerene unit (a conventional C
60
bearing a
methylene -CH
2
- laterally) substituted with a phenyl
group. C
6
H
5
on one side as well as with a butyric
acid ester - (CH
2
)
3
-COOH and methanol CH
3
OH,
forming, on the other side, a methyl butyrate group -
(CH
2
)
3
-COO-CH
3
.
It is extensively studied in the context of polymer
2,3 photovoltaic cells to form p-n junctions with
polythiophenes such as P3HT. As regards its
molecular weight, it is determined either by
osmometry, viscosimetry, quantitative and
qualitative chemical analyzes or by mass
spectrometry M(PCBM) = 910.8804 ± 0.0592 g/mol.
The PCBM is solubilized in chlorobenzene,
which results in its crystallization into nanocrystals
grouped together with different crystalline
orientations. This crystallization depends on the
nature of the solvent, in orthodichlorobenzene the
polymer crystallizes in the monoclinic system, in
contrast in chlorobenzene, it crystallizes in the
triclinic system that promotes optimal electron
transport. The covalent grafting of C
60
fullerene with
PCBM (necessary for more delocalization of
electrons) is carried out by, immersing it in a
ICCSRE 2018 - International Conference of Computer Science and Renewable Energies
220

solution of C
60
dissolved in benzene or toluene with
a concentration of 1 to 4 mmol /l. Then heating the
mixture under reflux under a nitrogen atmosphere
for 2 to 3 days. Then rinsing the samples obtained
with the pure solvent in order to remove the
physiosorbed C
60
molecules. The deposition of the
PCBM is carried out on the surface of a silica
substrate in order to measure the thickness by
ellipsometry, formed layers that can be calculated
from the following formula:
(6)
M: Number of oscillations.
: Wavelengths of the light passing
through the solution and the substrate.
and
indices of refractions of the
two mediums as a function of
.
The experimental measurement of the thickness
results in the value
. This shows that the
growth is carried out by 4 layers from bilayers.
Also the thickness can be measured by visible
UV absorption spectrophotometry by applying the
Beer-Lambert law:
(7)
Where A is the absorbance, α coefficient of
absorption of the layer vis-a-vis the radiation, ρ the
density of the layer, d the thickness of the studied
layer.
The covalent grafting between PCBM and C60
fullerene leads to changes in the absorption and
luminescence spectra of PCBMs that can be
characterized by Fourier Transform Infrared
Spectroscopy (FTIRS), so the evolution of the
substrate layer can be followed by surface-excited
Raman spectroscopy (SERS), this grafting is
manifested by the appearance of the characteristic
absorption bands in the infrared. For the visible UV
spectrum, we observe the appearance of a single
absorption band and this reveals a small amount of
C60 presented on the layers of PCBM. The quantity
of molecules on the surface remains constant as
shown by ESCA (electron spectroscopy for chemical
analysis) studies.
The stereochemistry of PCBMs is studied in 2D
((1H) and (13) C) NMR solutions in Deuterated
solvents (CDCl3, CD2Cl2) and the morphology of
the layer is characterized by Atomic Force
microscopy.
The mass percentages of C60 in the PCBM are
determined by thermogravimetric analysis from the
mass-temperature diagrams from which the different
degradation temperatures of the compound can be
derived. Concerning the P3HT crystallization
exotherms, the fusion endotherms are obtained by
the differential scanning calorimetry as well as the
melting and crystallization temperatures of the
P3HT:
and
The thermal effects of crystallization and
melting, the heating curves and those of the two
materials were studied by microcalorimetry(
and
) and the
differential thermal analysis, as well as the
crystallinity index of P3HT expressed by:
(8)
Or
: The enthalpy of fusion.
The reference enthalpy.
3 CHARACTERIZATION AND
ORGANIZATION OF THE
ACTIVE LAYER P3HT-PCBM
3.1 Organization of Polymer Chains
P3HT
The solubilization of P3HT in chlorobenzene is
dependent on the purity of the polymer, its high
molecular weight which can reach 50000 g / mol,
the stoichiometry, the chemical coupling conditions
(catalyst type, quantity and concentration) the
reaction conditions (1). absence of oxygen, good
magnetic or mechanical stirring) and finally the
solubility of the monomer and the polymers in the
reaction solvent. And in order to lead to a strong
fibrillar organization of the P3HT chains in the form
of a network leading to a high value of current
density, it is necessary that the interaction between
the polymer chains is more intense than their
interaction with the solvent. This aggregation of
chains in fibrillar structure is governed by
(π_stacking) and conditioned by the structure and
the chemical nature of the polymer, the interchain
interactions (hydrogen bonds, π interactions, dipolar
interactions...), solute solvent interactions and finally
the interactions of the substrate surface with the
polymer chains. The solvent of choice used for
structuring chains on fibrils is p-xylene.
(
)
which completely dissolves P3HT. At a temperature
of 80°C. For a concentration ranging from 0.5% (wt)
to 3% (wt) for one hour, which leads to an
organization of the chains or fibrils under low
temperature
for 4 hours in the
dark, the detection of these fibrils is carried out by
Physico-chemical Characterization, Structuration and Morphology of Photo-active Heterojunction (P3ht-Pcbm) Used In Organic
Photovoltaic Cells
221
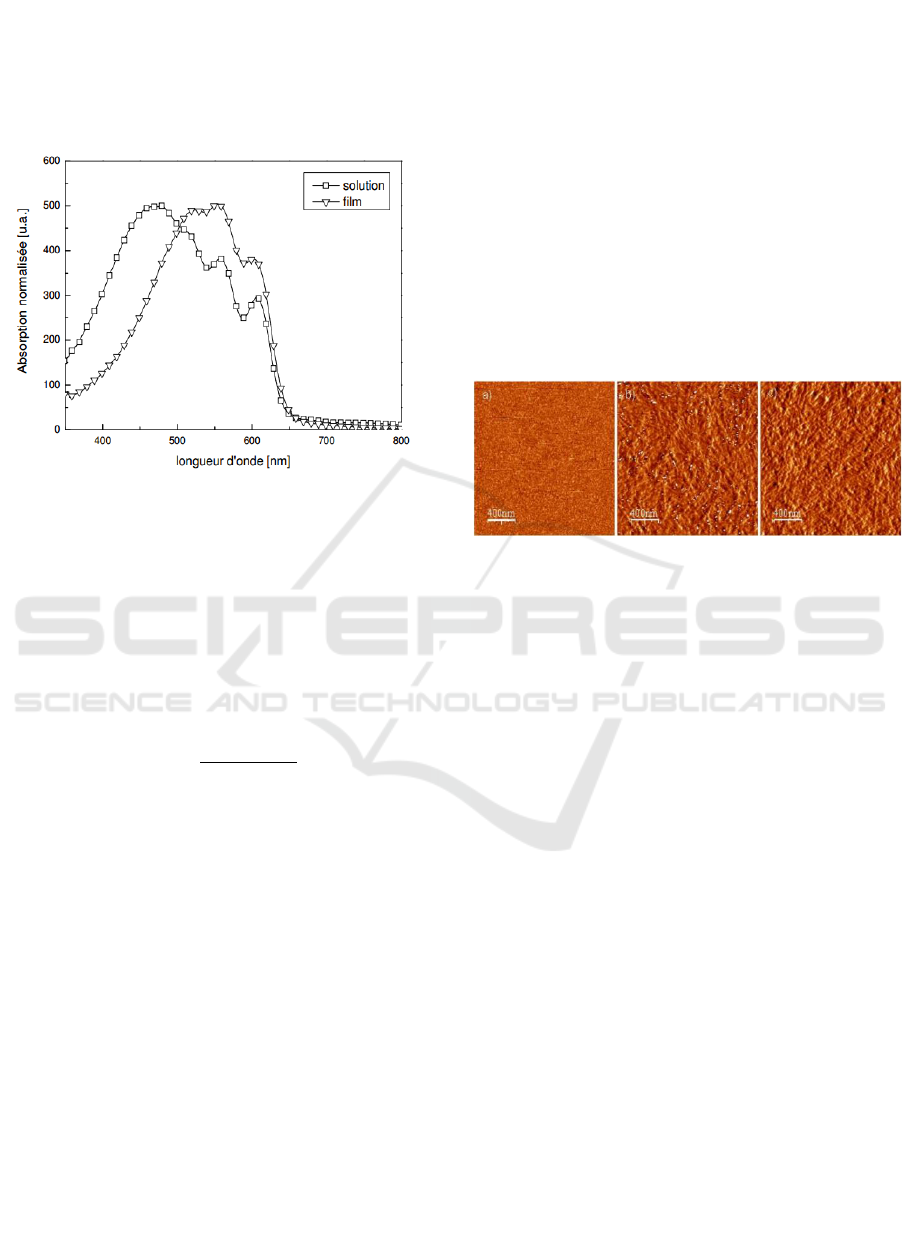
visible UV spectroscopy, we observe 3 absorption
bands 525 nm; 555nm; 610 nm at a concentration of
1% (wt) in p-xylene at a temperature of 80 ° C.,
identical to those of a P3HT film deposited by spin
caoting on a glass substrate for 2 h to 48 h with v =
20 ° C./h.
Figure 4: Normalized absorption spectra of a native
solution of P3HT fibrils in p-xylene and in film form.
This makes it possible to observe a good stack of
chains, thus a structure identical to that obtained in
the solid phase. An increase in the absorbance with
the fibril structuring explained by induced dipole-
induced dipole interactions, such as, for example,
London dispersion between the two chains of
interaction energy:
Donor oxidation potential (0.21V)
measured electrochemically (cyclic voltammetry) or
by UV and XPS spectroscopy (X-RAY PHOTO
ELECTRON SPECTOSCOPY)
: Anisotropic Polarisabilities of the two
chains.
ε: Dielectric constant of the solvent
r: The sum of the two Van-der-Waals radii of the
two chains
Eelementary charge
Number of transferred electrons
Interactions between fibrils can occur and result
in agglomeration of these which can be dispersed by
agitation of the solution.
Studies of the shape of these fibrils show their
lamellar organization in rod structure (measurement
of diffusion constant, flow birefringence,
sedimentation coefficient, electron microscopy,
coefficient of friction, radius of gyration, ...) On the
other hand, the transition from the disordered form
to the ordered form induces a bathochromic effect
which manifests a sol-gel transformation with an
isobestic point of 450 nm, thus an increase in the
regioregularity of the polymer, an optimization of
structuration chains requires the control of the
percentage of the fibrils in solution by means of
centrifugation followed by filtration and deposition
on the substrate by the dipping method or by the
spin-coating method and according to the
concentration used it is possible to obtain isolated
fibrils, fibrillar networks or complete layers of
morphology characterized by SEM (Scanning
Electron Microscopy) and AFM (Atomic Force
Microscopy): for the isolated fibrils found as
dimensions:
;
Figure 5: AFM images (non-contact tapping mode, phase
contrast image, 10x10 μm) obtained on P3HT films
obtained with (a) 0 wt%, (b) 75 wt% and (c) 97 wt% of
P3HT fibril in solution.
Thus, the presence of the non-regioregular poly-
3-alkylthiophene is observed, which leads to the
conclusion that the texture of the spin-on film on the
glass substrate coated with ITO (indium tin oxide)
which represents the anode, is determined by the
percentage of fibrils which is demonstrated by the
AFM, the higher this percentage is, the higher the
fibril surface concentration is important, this leads to
a controlled structuring which improves further by
heat treatment of P3HT (at 150 ° C. for 5 min)
which doubles crystalline domains and halves the
disorder, as shown by XRD( X-ray diffraction)
studies on films deposited on silicon substrates,
lamellar organization of fibrils with inter-lamellar
distance of 16.2 A ° and a coherence length l_c of
173 A ° are observed and this is due to the small
thickness of the lamellae, also the stacking
fluctuations are lower than those of the unannealed
P3HT, so the heat treatment results in the increase of
the regular order in the fibrils.
(9)
ICCSRE 2018 - International Conference of Computer Science and Renewable Energies
222

Figure 6: Simplified diagram of a regio regular P3HT
fibril.
Figure 7: Wide-angle X-ray diffraction spectra on films
spin coated on silicon substrates from non-fibrillar P3HT
before and after annealing and from fibrillar P3HT (native
solution).
Table 2: Position of diffraction peaks and associated
lattice spacings for a non-annealed P3HT film, the same
annealed film at 150 °C for 5 minutes and a fibrillar P3HT
film, obtained on spin-on silicon substrate.
Sample
Peak
number
Position [°]
Reticular
distance
[A°]
Disorder
(%)
width at half
height [rad]
Cohérence
length [A°]
P3HT not
annealed
001
002
003
6.22
12.78
18.96
16.48
8.04
5.43
9.5
6.7
5.5
0.013
0.047
0.091
96
P3HT
anneals 150
° C - 5 mn
001
002
003
6.22
12.39
18.55
16.49
8.30
5.55
4.5
3.2
2.6
0.0001
0.0002
0.0005
173
P3HT
fibril
001
002
003
6.44
12.82
19.23
15.93
8.01
5.36
4.7
3.3
2.7
0.0141
0.0200
0.0267
115
Finally, and to close with P3HT electrochemical
studies by cyclic voltammetry and electrochemical
impedance spectroscopy have shown that this
fibrillar organization modifies the potential for
oxidation-reduction of fibrilated P3HT as well as the
HOMO and LUMO energy. By cyclic voltammetry
at a scanning rate of 20 mV / s, two oxidation waves
are observed for the unstructured P3HT, which
means the presence of two forms: one structured
with low oxidation potential and the other
amorphous with high potential. oxidation. As
regards the fibrillated P3HT a single wave of
oxidation observed therefore the presence of a single
form.
Figure 8: Voltammograms on a gold electrode of a film of
P3HT and fibrillar P3HT (native fibril solution) obtained
by evaporation of gold electrode drop in 0.1M [Et 4NBF4]
/ PC, against electrode of platinum and reference Ag /
AgNO3 0.01 M in CH3CN at 20 mV.s-1.
There is a relationship between the energies of
the HOMO and LUMO orbitals and the threshold
redox potentials
these measures its calibrated
compared to the ferrocene
This three equations is very important because is
represent relations between quantum chemical
parameters
,
and electrochemical
parameters
and
.
(10)
(11)
(12)
Physico-chemical Characterization, Structuration and Morphology of Photo-active Heterojunction (P3ht-Pcbm) Used In Organic
Photovoltaic Cells
223

Table 3: Electrochemical characteristics obtained on a
gold electrode of a film of P3HT and of fibrillar P3HT
(native solution of fibrils) in 0.1M [Et4NBF4] / PC,
against platinum electrode and reference Ag / AgNO3 0,
01M in CH3CN. E (Fc/ Fc+ = 0.062 V) in PC.
Films
HOMO [eV]
LUMO [eV]
P3HT
0.206
-2.552
-4.94
-2.19
2.75
fibrillar
P3HT
0.239
-2.460
-5.00
-2.28
2.72
3.2 Organization of the Acceptor
Donor Active Layer (P3HT-PCBM)
Donor and acceptor were mixed together after
thermal annealing at 150 ° C for 5 min with the use
of the wrong solvent (chlorobenzene C= 10:10 mg /
ml) resulting in self-assembly of P3HT and PCBM
therefore the formation of a network of one
dimensional objects by induced dipole interactions
of the two polymers superior to the solvent-polymer
polarization interactions, thus an active layer with
donor-acceptor interfaces. The presence of PCBM
does not disturb the structured P3HT organization
but leads to the appearance of vibronic bands (515
nm, 551 nm, 603 nm) characterizing the formation
of the active layer that modify the absorption
spectrum of P3HT (hyperchromic effect).
The deposition of the layer on the ITO (Indium
tin oxide) anode which covers the glass substrate is
done by spin-coating at 2000 rpm for 2 min, the
cleaning of the substrate of the impurities is carried
out by ethanol under ultrasound. The aluminum
cathode is deposited by evaporation in vacuo and
results in a thickness ranging from 50 nm to 110 nm,
which improves the optical and electrical properties
of the cathode. Thermal or microwave treatment
allows segregation between polymers in the active
layer. The orientation of the grating effected by an
electric field perpendicular to the electrodes is due to
the anisotropy of the polarizability of the molecules.
The morphology of the active layer of P3HT_PCBM
after heat treatment is characterized by TEM
transmission electron microscopy.
Finally, the controlled morphology of the active
layer leads to the increase of the potential of the
open circuit V
OC
:
-0.3 V (11)
or
for the PCBM and
4 CONCLUSIONS
The use of different physicochemical techniques and
nanotechnology processes to characterize the
electron donor and acceptor polymers allows the
control at the nanometer scale of the morphology
and the texture of the active layer. Optimal
organization of the two materials P3HT and
PC61BM, in the acceptor donor heterojunction, thus
obtaining a privileged orientation of the molecular
fibrils of P3HT and nano-crystals of PC60BM with
respect to the electrodes, it is furthermore
advantageous to macroscopically transfer the This is
due to the stacking of the polymer chains and the
weak coupling between them, which further
optimizes inter-charge transfer at the expense of
intrachain transfer, which can hardly lead to a
macroscopic transfer of charge and thus to usable
electric currents.
And this is of course considered as a step in
advance of the molecular nanoelectronics that
eclipses day by day and that relegates in the shadows
conventional microelectronics derived from silicon
and inorganic semiconductors.
REFERENCES
Alet, P.-J. et al. (2006) ‘Hybrid solar cells based on thin-
film silicon and P3HT: A first step towards nano-
structured devices*’, The European Physical Journal
Applied Physics, 36(3), pp. 231–234. doi:
10.1051/epjap:2006145.
Baran, D. et al. (2017) ‘Reducing the efficiency–stability–
cost gap of organic photovoltaics with highly efficient
and stable small molecule acceptor ternary solar cells’,
Nature Materials, 16(3), pp. 363–369. doi:
10.1038/nmat4797.
Berson, S. et al. (2007) ‘Elaboration of P3HT/CNT/PCBM
Composites for Organic Photovoltaic Cells’, Advanced
Functional Materials, 17(16), pp. 3363–3370. doi:
10.1002/adfm.200700438.
Björström, C. M. et al. (2005) ‘Multilayer formation in
spin-coated thin films of low-bandgap
polyfluorene:PCBM blends’, Journal of Physics:
Condensed Matter, 17(50), pp. L529–L534. doi:
10.1088/0953-8984/17/50/L01.
ICCSRE 2018 - International Conference of Computer Science and Renewable Energies
224

Brabec, C. J. et al. (2010) ‘Polymer-Fullerene Bulk-
Heterojunction Solar Cells’, Advanced Materials,
22(34), pp. 3839–3856. doi:
10.1002/adma.200903697.
Chen, D. et al. (2011) ‘P3HT/PCBM Bulk Heterojunction
Organic Photovoltaics: Correlating Efficiency and
Morphology’, Nano Letters, 11(2), pp. 561–567. doi:
10.1021/nl103482n.
Dang, M. T., Hirsch, L. and Wantz, G. (2011)
‘P3HT:PCBM, Best Seller in Polymer Photovoltaic
Research’, Advanced Materials, 23(31), pp. 3597–
3602. doi: 10.1002/adma.201100792.
Destruel, P. and Seguy, I. (2007) ‘Les cellules
photovoltaïques organiques’, Reflets de la physique,
(6), pp. 16–18. doi: 10.1051/refdp/2007064.
Peumans, P., Yakimov, A. and Forrest, S. R. (2003)
‘Small molecular weight organic thin-film
photodetectors and solar cells’, Journal of Applied
Physics, 93(7), pp. 3693–3723. doi:
10.1063/1.1534621.
Rispens, M. T. et al. (2003) ‘Influence of the solvent on
the crystal structure of PCBM and the efficiency of
MDMO-PPV:PCBM “plastic” solar cells’, Chem.
Commun., (17), pp. 2116–2118. doi:
10.1039/B305988J.
Vanlaeke, P. et al. (2006) ‘P3HT/PCBM bulk
heterojunction solar cells: Relation between
morphology and electro-optical characteristics’, Solar
Energy Materials and Solar Cells, 90(14), pp. 2150–
2158. doi: 10.1016/j.solmat.2006.02.010.
Physico-chemical Characterization, Structuration and Morphology of Photo-active Heterojunction (P3ht-Pcbm) Used In Organic
Photovoltaic Cells
225
