
Effect of Addition Mg and Na on Phase Formation and Crystallite
Size of BPSCCO-2223 Superconductor
Syahrul Humaidi
1
, Wahyu Azhar R.
1
,Timbangen Sembiring
1
and Agung Imaduddin
2
1
Physics Department, FMIPA, Universitas Sumatera Utara, Jln Bioteknologi no 1 Medan 20155, Indonesia
2
Research Center for Metallurgy and Materials, Indonesian Institute of Science, PUSPITEK, Tangsel 15314, Indonesia
Keywords: BPSCCO-2223, Superconductors, powder, Tc
zero
Abstract: Superconductor samples based on BPSCCO-2223 have been prepared using solid state reaction. Precursor
material (powder): Bi
2
O
3
, PbO
2
, SrCO
3
, CaCO
3
and CuO were mixed together using agate mortar for 2 h
with twice grindings. Powder were then sintered at 300°C for 6 h with a rate of 10°C/min and 820°C for 20
hours at the same rate. MgO powder was added for 5%wt and 15%wt, respectively. The same amount as
well as same procedure was applied for Na
2
CO
3
powder addition. The powder was then put into palletising
machine with 250 MPa before sintering at 850°C for 30 hours. Effect of addition Na and Mg on crystallite
size and phase formation had been observed using XRD (powder method) type PAN analytical Empyrean.
XRD-pattern was analysed with aid of Match v1.10 software. Results showed that crystallite size increased
for 9.5% by addition of 5%wt Mg but decreased for 13.6% with 15%wt Mg. Addition of 5%wt Na
decreased the crystallite size for 31.8%, whereas 15%wt Na decreased it to 14.2%. Maximum volume
fraction of Bi-2223 (65.18%) was observed in non-doped sample.
1 INTRODUCTION
Superconductors generally classified as low
temperature and high temperature depend on their
critical temperature. Since the discovery of
superconductor, many researches have been
developing this kind of material around the word.
Superconductor materials Bi-based become an
interesting and promising material since non toxic,
inherently high critical temperature and multi
phases. There are many techniques in preparation of
Bi-based superconductor. The effective method is a
method of solid state reaction, a mixture of oxide,
peroxide and nitrate (
R Abd-Shukor, 2004; R H Patel et
al., 2005).
Bismuth Strontium Calcium Copper
Oxide or BSCCO (bisco) can be categorized as a
high temperature superconductor with formula of
Bi
2
Sr
2
Ca
n-1
Cu
n
O
2n+4+x
. Many researches have been
done for n=2, whereas for n=1 and n=3 are also
interesting materials to develop. BSCCO is similar
with YBCO superconductor.
BSCCO depends on number of its metallic ion.
Thus, Bi-2201 is associated with n=1 (Bi
2
Sr
2
CuO
6+x
), Bi-2212 with n=2 (Bi
2
Sr
2
CaCu
2
O
8+x
) and
Bi-2223 for n=3 (Bi
2
Sr
2
Ca
2
Cu
3
O
10+x
). Some system
have been investigated such as: Bi-2201 (Tc=20K),
Bi-2212 (Tc=95K), Bi-2223 (Tc=108K) and Bi-
2234 (Tc=104K). Modifications have also been
made with Pb substitution (
Abbas et al., 2015). It was
found that Pb stabilized the structure. MgO addition could
increase
Bi-2223 phase as reported by Lu (2016). Na
could increase critical temperature (Kir Ebru, 2016).
Effect of Sb doped Bi-based superconductor reported by
Hermiz (2015).
It has been known that rare-earth elements
improve the phase formation of the Tl-based
superconductors (Syahrul Humaidi, 2019). In this
brief paper we report on the effect of Mg and Na on
the superconducting properties and phases formation
of the BPSCCO-2223 phase.
2 EXPERIMENTAL
The samples were prepared using solid state
reaction. To start with, powder with high purity of
Bi
2
O
3
(powder), PbO
2
, SrCO
3
, CaCO
3
, CuO, MgO and
Na
2
CO
3
. All oxides are in the powder preparation. The
precursor BPSCCO was synthesized with two times
grindings for 2h before sintering at 820°C for 20h.
Mg and Na were added in final step of grinding. The
next step was palletizing process at 70 ton to prepare
Humaidi, S., Azhar R., W., Sembiring, T. and Imaduddin, A.
Effect of Addition Mg and Na on Phase Formation and Crystallite Size of BPSCCO-2223 Superconductor.
DOI: 10.5220/0010181000002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 319-321
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
319
pellet sample and followed by sintering process at
temperature of 850°C for 30h. Characterization of
samples cover: resistivity using cryogenics to obtain
critical temperature, T
c zero
(four point probe method;
XRD analysis using MATH computer program.
Based on cryogenics and XRD analysis, crystallite
size and critical temperature of the samples can be
obtained as presented in Table 1.
Table 1: Critical temperature and crystallite size
N
o Sample T
c zero
d (nm)
1
N
on-dope
d
86K 56.55571
2 5%M
g
O 111K 61.94372
3 15%M
g
O 108K 48.87306
4 5%Na 80K 38.59899
5 15%Na 75K 48.53214
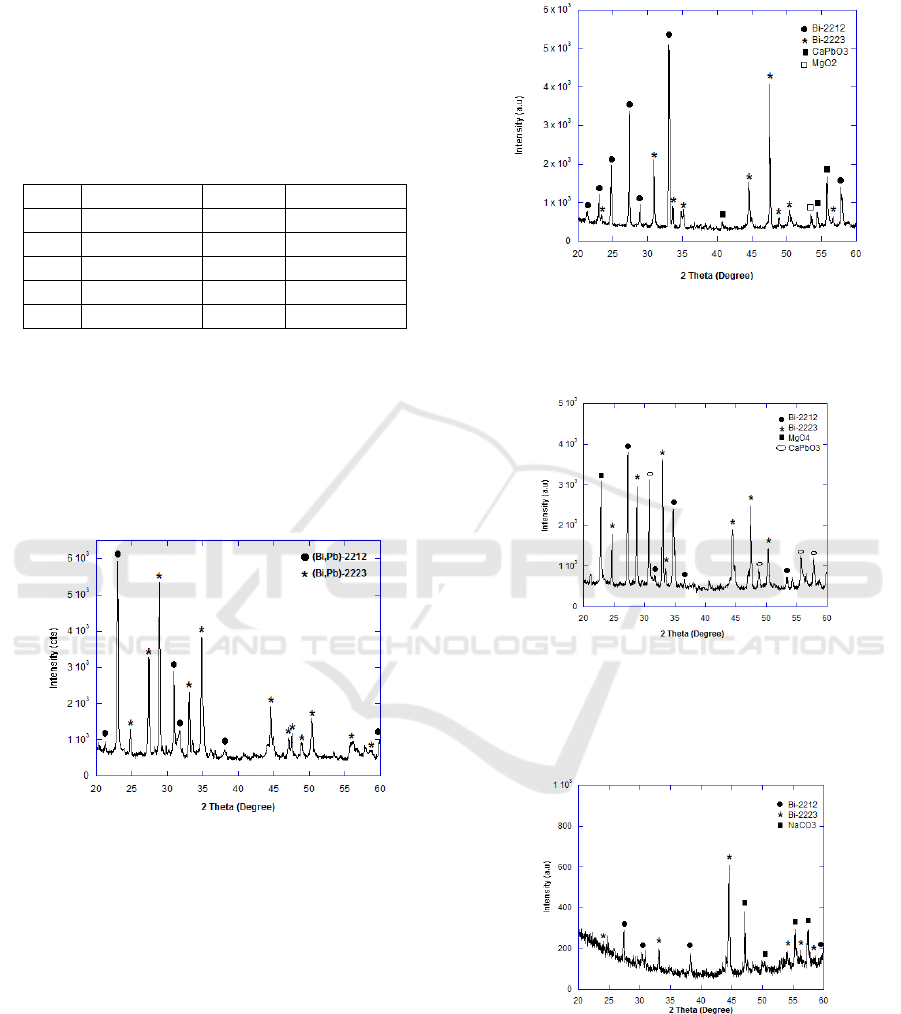
XRD-pattern of samples are presented in Figure
1- Figure 5. Figure 1 shows XRD-pattern of non-
doped Bi
1.6
Pb
0.4
Sr
2
Ca
2
Cu
3
O
10+δ
sintered for 30h at
temperature of 850°C. The occurrence of BPSCCO-
2223 phase is around 66% denoted as (*) as
presented in Figure 1. The rest phase is BPSCCO-
2212 for 36%.
Figure 1: XRD-pattern for Bi
1.6
Pb
0.4
Sr
2
Ca
2
Cu
3
O
10+δ
.
Based on Figure 1, the space system of BPSCCO-
2223 is orthorhombic with a=5.4056Å. In can be
noted that no other impurity phase detected in this
sample. The history of phase formation when 5%wt
Mg was introduced can be seen in Figure 2. Based
on XRD analysis, the lattice parameter a= 5.4056 Å,
b= 5.4055 Å, c= 37.12, respectively. Phase
formation of BPSCCO increases to 55% when the
Mg content increase up to 15%wt as presented in
Figure 3. XRD-pattern of 5%wt Na as shown in
Figure 4. Like the addition of Mg, impurities have
been detected in Na-doped as tabulated in Table 2.
Phase formation of BPSCCO increases to 55% when
the Mg content increase up to 15%wt as presented in
Figure 3. Figure 4 and Figure 5 show the XRD-
pattern of Na-doped BPSCCO.
Figure 2: XRD-pattern of BPSCCO
+ Mg 5%wt
It is interesting to note that the phase formation
affected the critical temperature of the samples.
Figure 3: XRD-pattern of BPSCCO
+ Mg15%wt
Phase formation of BPSCCO increases to 55%
when the Mg content increase up to 15%wt as
presented in Figure 3.
Figure 4: XRD-pattern of BPSCCO
+ Na5%wt
The effect of addition Mg and Na on phase
occurrence can be summarized in Table 2.
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
320
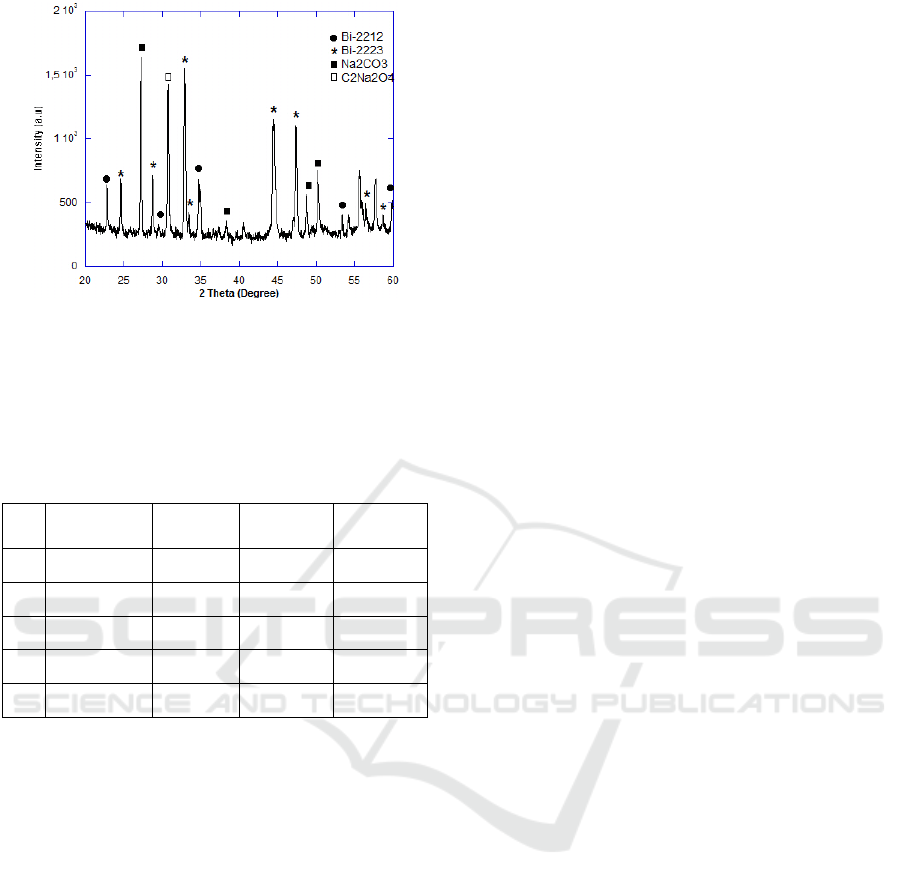
Figure 5: XRD-pattern of BPSCCO
+ Na15%wt
As it can clear be seen, no other impurity phase
present in non-doped sample. However the content
of impurities increases to 13% when 5%wt Mg was
introduced to precursor material.
Table 2: Phase formation of BPSCCO
No T
sint
/t
Doped
with
BPSCCO
(2212)
BPSCCO
(2223)
1 850°C/30h 0% 34% 65%
2 850°C/30h Mg5% 46% 41%
3 850°C/30h M
g
15% 24% 55%
4 850°C/30h Na5% 19% 42%
5 850°C/30h Na15% 11% 45%
The addition of 15% Mg increased the impurities
content for 21%. The presence of impurities as a
result of imperfection chemical reaction took place
during the sintering. The same result can be noted in
addition of Na. As it can be seen, impurities content
increased for 31% and 39% when Na content 5%
and 15%, respectively. It can be concluded that the
effective doping level was in a small amount of Mg
and Na.
4 CONCLUSIONS
Superconductor material Bi-based have been
prepared with BPSCCO-2223 as major phase.
Addition Mg and Na affect the superconductor
parameters. Crystallite size as well T
c zero
decreased
as Mg and Na content increase. Impurities
occurrence decrease the zero critical temperature.
REFERENCES
Hermiz G Y, Jassim A K, Oboudi S F, 2015, Electrical,
Structure and Morphological Properties of Sb-Doped
Bi-Based Superconductor. Advanced in Material
Physics and Chemistry 5:214-220
Humaidi, Syahrul, Muhammad Razali and Roslan Abd-
Shukor, 2018, Preparation of Tl-2212 Cr-Subst. (Tl
2-
x
Cr
x
)Ba
2
CaCu
2
O
8-d
Superconductor, Journal of
Physics: Conf. Series 1116 032010
Kir Ebru, 2016, The Effect of K-Na-co-doping on the
formation and particle size of Bi-2212 phase. Physica
B. DOI: 10.1016/j.physb.2016.03.016
Lu X Y, Yi D, Chen H, Nagata A, 2016, Effect of Sn,
MgO and Ag
2
O mix-doping on the formation &
superconducting properties of Bi-2223 Ag/tapes.
Physics Procedia 81:129-132
Monshi, A., Foroughi, M.R.,Monshi, M.R, 2012, Modified
Scherrer Equation to Estimate More Accurately Nano-
Crystallite Using XRD. World Journal of Nano
Science and Engin., 2:154-160.
R Abd-Shukor, 2004, Introduction to Superconductivity:
in Metals, Alloys & Cuprates, Universiti Pendidikan
Sultan Idris Publisher, Tanjong Malim, Malaysia.
R H Patel, A Nabialek and M Niewczas, 2005,
Characterization of superconducting properties of
BSCCO powder prepared by attrition milling,
Supercond Sci Tech 18 317-324, IOP
Syahrul Humaidi, Eddy Marlianto, S. Marhaposan, and R.
Abd-Shukor, 2019, Superconducting Properties of Te-
Subst. (Tl
2-x
Te
x
)Ba
2
CaCu
2
O
8-δ
, Solid State
Phenomena, Vol.290(23), pp 239-244
Effect of Addition Mg and Na on Phase Formation and Crystallite Size of BPSCCO-2223 Superconductor
321
