
New Photonic Materials based on Ag Nanoparticles Modified with
Stilbene Dyes and Its Peculiar Behavior Studied with SERS
Alexey N. Smirnov, Olga V. Odintsova and Elena V. Solovyeva
Chemistry Institute, Saint-Petersburg State University, 26 Universitetsky pr., Peterhof, Saint-Petersburg, Russia
Keywords: SERS, Raman, Stilbenes, Amines, Hot Spots, Ag Nanoparticles.
Abstract: In the recent years, there has been a growing interest in development of smart photoactive materials with
variable properties. This work presents the SERS study of behaviour of organometallic composites obtained
by modification of Ag nanoparticles in hydrosols with several amino derivatives of stilbene. The feasibility
of bifunctional stilbenes to be the molecular linkers of Ag nanoparticles is discussed. The hot spots activity
of such modified Ag nanoparticles is considered. Two exciting but not fully understood finding were made:
i) tertiary amines can be used as the effective molecular linkers; ii) the structure of central fragment between
two benzyl rings has a strong influence on the modifier ability to incorporate the Ag nanoparticles into
agglomerates with hot spots. The limitations consisting in the pH value and the presence of chloride ions are
described for a potential application of the developed hot spots substrates.
1 INTRODUCTION
Development of new photonic materials is a subject
of great interest in modern physics, chemistry and
materials science. Integration of photoactive
molecules with plasmonic nanostructures represents
one of the most promising approach for engineering
such materials. Among the plasmonic
nanostructures, the substrates with hot spots are
particularly attractive. Hot spots are the regions
between closely located nanoroughnesses where a
superposition of local electromagnetic fields occurs
(Schlücker, 2014; Roelli 2016). Inside the hot spots
greater enhancement of optical signal, e.g. Raman
scattering, is provided. Hot spots can be obtained by
self-assembling nanoparticles using molecular
linkers. The term “molecular linkers” denotes the
molecules which have a capability of simultaneous
attachment to two different surfaces. Due to higher
optical response from hot spots, among the
photoactive molecules able to electrostatically or
chemically interact with a substrate the choice is
preferable for bifunctional ones.
In this study, we present the investigation of
organometallic composites based on Ag
nanoparticles (Ag NP) modified with
aminostilbenes. This class of organic pigments has
the pronounced photoactivity (Su, 2017; Liu, 2000).
A combination of aminostilbenes photophysical
properties with the plasmonic properties of Ag NP is
promising strategy for obtaining the systems whose
optical properties can be controlled by external
forces. In our previous work, we focused on 4,4'-
diaminostilbene and its ability to link the metal
nanoparticles was demonstrated (Solovyeva, 2018).
In the present study, the particular attention was paid
to exploring an influence of dye’s structure on the
molecule-surface interaction. Three dyes differing in
the functional groups and saturation of central bond
(see Fig. 1) were investigated in silver hydrosols by
surface enhanced Raman spectroscopy (SERS) and
transmission electron microscopy (TEM). It was also
importantly to evaluate a behavior of obtained
systems under changing conditions. Such parameters
as pH or presence of chloride anions may be crucial
for electric double layer structure and Ag NP –
molecule interaction consequently.
Figure 1: Common chemical structure of investigated
compounds. 1a – DAS; 1b – ADMAS; 1c – AS, 2 –
DABB (meaning of abbreviations see in Experimental).
Smirnov, A., Odintsova, O. and Solovyeva, E.
New Photonic Materials based on Ag Nanoparticles Modified with Stilbene Dyes and Its Peculiar Behavior Studied with SERS.
DOI: 10.5220/0007569302630267
In Proceedings of the 7th International Conference on Photonics, Optics and Laser Technology (PHOTOPTICS 2019), pages 263-267
ISBN: 978-989-758-364-3
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
263

2 EXPERIMENTAL
4-aminostilbene (AS) (98%), 4,4'-diaminostilbene
dihydrocloride (DAS) (95%), 4-amino-4'-(N,N-
dimethylamino)stilbene (ADMAS) (98%) and 4,4'-
diaminobibenzyl (DABB) (95%), were purchased
from Sigma-Aldrich. DAS was recrystallized in
MeOH before using. Other compounds were used
without further purification. Methanol solutions of
AS and ADMAS with concentration in the range
from 1×10
-7
to 1×10
-5
M were prepared from stock
solution (1×10
-4
M) by volume dilution method for
SERS measurements. Silver hydrosol was prepared
by reduction of silver nitrate by sodium borohydride
in accordance with the standard procedure. To
decrease pH of solution, nitric acid with appropriate
concentration was used. To provide the halide
anions effect, potassium chloride was added in silver
hydrosol.
The SERS spectra were recorded using LabRam
HR800 (Horiba Jobin Yvon) spectrometer with CCD
detector. The incident laser excitation was 488 nm
line from Ar
+
laser source. Laser power at a sample
was 20 mW. All SERS spectra were registered in the
range of 400-1800 cm
-1
, in four acquisitions, 20 s
accumulations. The images of Ag nanoparticles were
obtained with a Zeiss Libra 200FE transmission
electron microscope (TEM) at an accelerating
voltage of 200 kV. TEM images in scanning mode
(STEM) were taken from at least three random
domains of the sample. In order to prepare samples
for TEM measurements, 10 µl of silver hydrosol
were drop casted on top of carbon films and air
dried. During solvent evaporation the films were
kept in a dark place.
3 RESULTS ANS DISCUSSION
A concentration of organic additives in some cases
has a crucial influence on the properties of metal
hydrosols. Therefore, a wide range of concentrations
has to be considered for firstly applied modifiers.
Two types of concentration dependence of SERS
spectra were found for the studied stilbene
derivatives. As one can see from Fig. 2, the
monotonous growth of SERS signal along with the
concentration is observed for AS. While the non-
monotonic change of SERS intensity together with
the significant transformation of spectral profile can
be seen for ADMAS. The growth of intensity
follows to the increase of ADMAS concentration up
to 1 × 10
-6
M. The drop of SERS signal is further
observed proceeding up to 1 × 10
-5
M. The same
type of SERS spectra dependence on concentration
was obtained for DAS in our previous study
(Solovyeva, 2017).
600 1200 1300 1400 1500 1600 1700
AS
ADMAS
1E-7 5E-7 1E-6 5E-6 1E-5
Raman shift (cm
-1
)
Figure 2: Dependence of SERS spectra on the
concentration (M) of ADMAS and AS in silver hydrosol.
The monotonous increase of SERS intensity in
case of AS corresponds to conventional gradual
filling of surface-solution interface without a
substantial alteration of molecular layers
configuration and surface morphology. The behavior
of ADMAS spectra similar with DAS suggests that
the same adsorption phenomena produce the
observed spectral changes. When the first monolayer
becomes completed, the way of molecules
interaction with surface changed. Obviously, this
happens at 1 × 10
-6
M and, thus, produces the
observed intensity drop and transformations of
spectral profile. At sub-monolayer adsorption,
ADMAS, as a bifunctional molecular linker,
interacts simultaneously with two different
nanoparticles by donation of nitrogens lone pairs. In
such conditions, ADMAS molecules are located in
the hot spots and its Raman scattering undergoes to
higher enhancement. At multilayer adsorption,
linking the nanoparticles via ADMAS becomes
impossible that leads to the ordinary Raman
enhancement.
The proposed hypothesis is consistent with the
data obtained by scanning transmission electron
microscopy for the same systems (fig 3). In the
presence of AS at any concentration, the
nanoparticles size and morphology do not change
PHOTOPTICS 2019 - 7th International Conference on Photonics, Optics and Laser Technology
264

significantly in comparison with the pure silver
hydrosol. In case of ADMAS, the oblong
agglomerates, which look like the glued
nanoparticles, can be seen in the TEM images
obtained for hydrosol with 1 × 10
-6
M of modifier.
For solution with 1 × 10
-5
M of ADMAS, the TEM
showed the small spherical nanoparticles as are
typical for borohydride-reduced silver hydrosol.
Figure 3: TEM images of Ag NPs modified by AS and
ADMAS at different concentrations.
Thus, we can conclude that the methyl
substitutes in one of two amino groups of ADMAS
do not obstruct to its coordination with surface.
Thereby, ADMAS has the same linking ability as
DAS. Such results are quite surprising but they mean
that a list of compounds, which can be used as
molecular linkers, can be significantly expanded.
The corresponding adsorption models are depicted at
fig. 4.
The solutions of AS and ADMAS in silver
hydrosol were further investigated in various
environments to get a deeper insight into adsorption
1200 1400 1600
1200 1400 1600
5000 a.u.
Raman shift (cm
-1
)
AS
40000 a.u.
ADMAS
Figure 4: Schematic models of adsorption for mono- and
bifunctional stilbene dyes at 1 × 10
-6
M conc. at Ag
nanoparticles in hydrosol.
mechanisms and to evaluate the limits for practical
applications of modified Ag NPs. The SERS spectra
of stilbene derivatives at neutral and acidic pH are
presented as Fig. 5. For ADMAS the effect of
solution acidification is examined at various
concentrations corresponding to two different
adsorption modes. The most substantial influence of
pH is observed for ADMAS at concentration of 1 ×
10
-6
M, when the molecules are attached to the two
different nanoparticles. In this case, the intense
SERS spectrum transforms to the spectrum of lower
intensity, and the noticeable bands shifts can be seen
also. It is should be noted that the profile of
spectrum at 1 × 10
-6
M ADMAS after acid addition
converts to the profile similar with those observed
New Photonic Materials based on Ag Nanoparticles Modified with Stilbene Dyes and Its Peculiar Behavior Studied with SERS
265

for 1 × 10
-5
M ADMAS at pH 2. Apparently,
molecules of ADMAS become disabled to «glue»
the nanoparticles after protonation of amino
(dimethyl) group. Protonated ADMAS molecules
adsorb on the surface in the same way whether sub-
monolayer or multilayer coverage. The changes in
the spectral profile are caused by electron density
redistribution. This is highlighted by the fact the
most significant profile change is the intensity
increase of modes near 1369 cm
-1
relating to the
deformational vibrations of double bond (Solovyeva,
2019). Probably, a reorientation of molecules
induced by protonation also takes place.
600 1200 1300 1400 1500 1600 1700
Raman shift (cm
-1
)
ADMAS
1E-6 M
pH 7 pH 2
AS
1E-5 M
ADMAS
1E-5 M
Figure 5: Dependence of Raman signal from pH for
ADMAS and AS 10
-6
and 10
-5
M solutions in Ag
hydrosol.
The halide anions have a high affinity to silver
surface. The study of organics in metal hydrosols in
their presence allows one to evaluate the processes
of competitive adsorption. To this end, a potassium
chloride was added to the prepared organometallic
systems. Fig. 6 represents the effect of chloride
anions on the SERS spectra of AS and ADMAS. As
can be seen, the dramatic changes of SERS spectrum
occur for ADMAS at low concentrations
corresponding to sub-monolayer adsorption. In case
of AS, as well as for ADMAS at multilayer
adsorption, the addition of chloride ions is followed
only by moderate increase of intensity and some
bands transformations in their spectra. Apparently,
chloride ions, embedding in the double electric
layer, displace the ADMAS molecules from the first
monolayer. This leads to the observation of ADMAS
spectra characteristic for multilayer adsorption when
the SERS signal is collected from several layers,
including the top layers. It is should be mentioned,
that the observations for ADMAS at protonation and
chloride addition are the same with those that were
revealed for DAS.
600 1200 1300 1400 1500 1600 1700
ADMAS
1E-6 M
ADMAS
1E-5 M
Raman shift (cm
-1
)
without KCl 4.31E-2KCl
AS
1E-5 M
Figure 6: Change of Raman signal for AS and ADMAS
after addition of chloride ions in Ag hydrosol.
A short time ago, we have investigated an ability
to link the Ag nanoparticles for bifunctional DABB,
which distinguishes from the stilbene derivatives by
the structure of central fragment. In DABB, the
benzyl rings are bonded with each other by the
saturated ethane fragment instead of the double bond
in stilbenes (see fig. 1).
Unexpectedly, the SERS spectra of DABB have
demonstrated the monotonous dependence on the
PHOTOPTICS 2019 - 7th International Conference on Photonics, Optics and Laser Technology
266
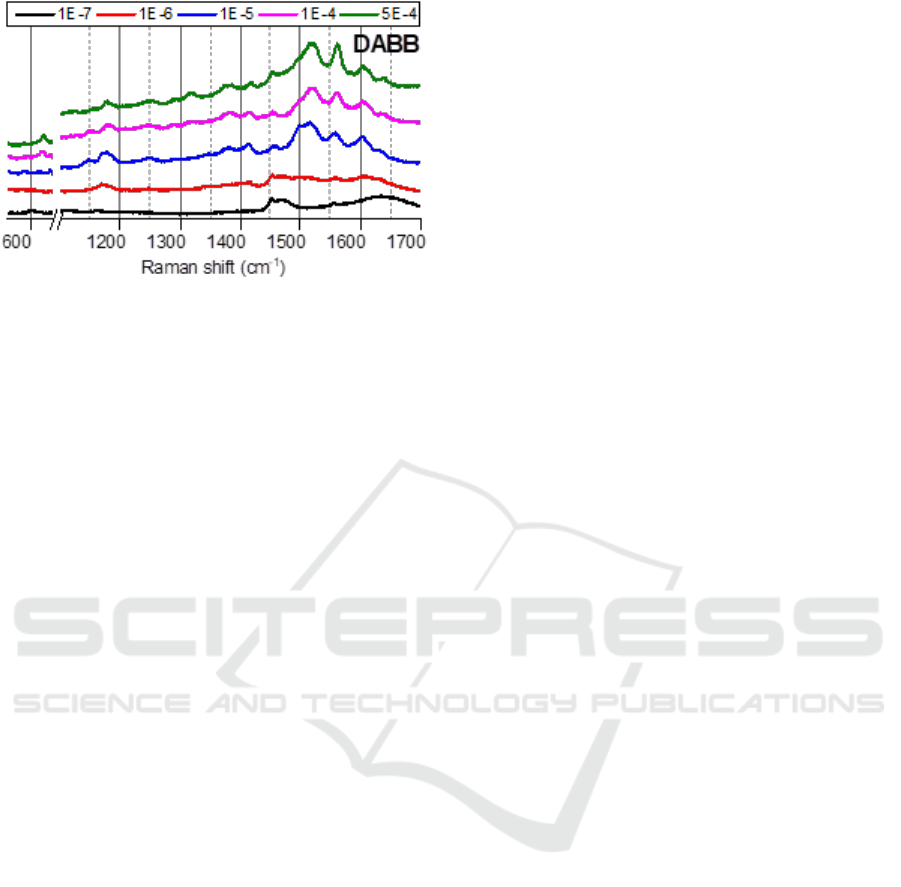
Figure 7: Dependence of SERS spectra of DABB on
concentration (M) in silver hydrosol.
concentration similar with AS (fig. 7). This suggests
that DABB do not link Ag NPs despite of two amino
groups. No color change observed for the
corresponding solutions of DABB in silver hydrosol
also implies that the Ag nanoparticles save their size.
This supports the previous assumption about
inability of DABB to modify Ag NPs into
agglomerates with hot spots. Obviously, the
saturation of central fragment, defining a
conjugation in the whole molecule, has a key
significance for linking ability of studied aromatic
amines. Revealing a role of central fragment
structure in the interaction of molecules with the
metal nanoparticles will be a subject of our future
investigation.
4 CONCLUSIONS
Based on the obtained results, we proposed the
models of adsorption for investigated stilbene dyes
(fig. 4). At sub-monolayer adsorption, bifunctional
aminostilbenes coordinate with the surface of two
Ag nanoparticles simultaneously that leads to the
formation of agglomerates with hot spots. The
stilbene derivatives with one amino group adsorb on
Ag nanoparticles by ordinary layer-by-layer way.
The bifunctional stilbenes with the tertiary amines
are also able to bind the metal nanoparticles, despite
the fact that tertiary amines are less active electron
density donors than primary ones.
Thanks to that, a list of photoactive compounds,
which can be used as promising modifiers of
plasmon substrates, can be significantly expanded.
However, the efficiency of bifunctional stilbenes
dyes as molecular linkers is lost in acidic solutions
and at the presence of chloride ions. At the same
time, the surface properties of single-particle
organometallic systems change due to the
redistribution of potential energy. The comparative
SERS study of bifunctional stilbenes and bibenzyls
showed that a conjugation in the molecule is also
significant for ability to link the nanoparticles.
The results of present study are able to find the
application points in the broad range of modern
research and development areas, connected to
photoactive materials. The revealed SERS response
of obtained organometallic composites dependent on
the molecular structure and concentration of
modifier as well on the environmental parameters
gives the deeper insight into surface chemistry and
practical possibilities of these and similar systems.
ACKNOWLEDGEMENTS
This work was supported by the Russian Science
Foundation (grant № 17-73-10209).
The experimental data was obtained using the
equipment of the Resources Centres of Saint-
Petersburg State University. The SERS spectra were
collected in the Center for Optical and Laser
Materials Research. The TEM images were obtained
in the Interdisciplinary Center for Nanotechnology.
REFERENCES
Schlücker, S. 2014. Angew. Chemie Int. Ed., 53(19),
4756–4795.
Roelli, P. et al. 2016. Nat. Nanotechnol., 11(2), 164–169.
Su, J. et al. 2017. Dyes and Pigments., 146, 92-102.
Liu C. et al. 2000. J. of Mol. Struct. (Theochem) 531, 169-
174.
Solovyeva E.V., Ubyivovk E.V., Denisova A.S., 2018.
Coll. Surf. A., 528, 542-548.
Solovyeva, E. V. et al. 2019. J. Mol. Struct., 1175, 287–
296.
New Photonic Materials based on Ag Nanoparticles Modified with Stilbene Dyes and Its Peculiar Behavior Studied with SERS
267
