
Acidic and Basic Amino Acids Gasification Characteristics under
Supercritical Water Conditions
Thachanan Samanmuly
1,*
and Yukihiko Matsumura
2
1
Department of Chemical Engineering, Faculty of Engineering, King Mongkut’s Institute of Technology Ladkrabang,
Chalongkrung Road, Ladkrabang, Bangkok 10520 Thailand
2
Division of Energy and Environmental Engineering, Institute of Engineering, Hiroshima University, 1-4-1 Kagamiyama,
Higashi-Hiroshima, 739-8527 Japan
Keywords: Amino acids, Hydrothermal, Supercritical water, Gasification, Biomass
Abstract: Animal biomass wastes and aquatic biomass are alternative biomass materials for renewable energy
production which are contained low or no lignocellulosic. Protein is one of major contents in food waste,
animal matter, and algae. The 2 selected amino acids, glutamic acid and arginine, were chosen to determine
the gasification characteristics. Aqueous solution 1.0 wt% of those two amino acids was gasified under
supercritical water conditions by using a tubular flow reactor. Reaction temperatures were varied ranging
between 500 and 650 oC and pressure was fixed at 25 MPa. Aqueous feedstock flow rate also fixed at 2.0
g/mL for a residence time of 86-119 s. Identification and quantification of the gas products were examined
by gas chromatography (GC). The aqueous phase product was also determined the dissolved carbon by the
total organic carbon (TOC) analyzer. An increasing reaction temperature improved the carbon gasification
efficiency. The gasification rate of glutamic acid and arginine follow Arrhenius behaviors and are
explained well by the first order kinetics equation. Gasification characteristics of glutamic acid and arginine
were also compared to those of glycine and alanine. The effect of the functional group in arginine is an
increasing alkalinity that made pH of liquid products are above 9.
1 INTRODUCTION
Nowadays, the world energy consumption is
tendency rises with the rising of fossil fuel depletion
and the global environmental problem. Then,
renewable energy such as biomass-derived energy
becomes an attractive issue to reduce the fossil fuel
production and develop to be a sustainable energy
resource. Generally, organic compounds in biomass
cannot be dissolved in normal conditions water due
to they are non-polar molecules. Under supercritical
conditions (Tc = 647 K or 374
o
C, Pc = 22.1 MPa),
supercritical water became a better solvent more
than a normal conditions water that behaves like an
organic solvent. Supercritical water dissolved
organic compounds, the lignocellulosic compounds,
polysaccharide, and protein, which presented in
biomass and hydrolyzed to form glucose, xylose,
amino acids, and organic acids which are further
utilized as feedstocks of bio-ethanol or bio-
chemicals or bio-fuel productions. Then,
supercritical water gasification has excellent
reactivity, which makes it a very promising reaction
medium for converting various types of biomass into
value-added fuel products. Furthermore,
supercritical water gasification has been developed
not only to solve the problem of tar and char
formation which initiated low conversion efficiency
and reactor plugging but also increases gasification
efficiency (Antal et al., 2000).
Many works have studied and revealed that
supercritical water gasification technology is an
innovative thermochemical methodology for
converting wet biomass and organic waste into
combustible gases, such as hydrogen and methane
(Matsumura et al., 2013). A wide variety of model
biomass compounds have been separately gasified in
supercritical water in order to investigate the
gasification characteristics of substantial biomass
species as the representative of real biomass
containing these compounds. But, the gasification
rate of specific feedstocks is still miserable to
Samanmuly, T. and Matsumura, Y.
Acidic and Basic Amino Acids Gasification Characteristics under Supercritical Water Conditions.
DOI: 10.5220/0008652500170022
In Proceedings of the International Conference on Future Environment Pollution and Prevention (ICFEPP 2019), pages 17-22
ISBN: 978-989-758-394-0
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
17

predict. There are not much known about the
gasification characteristics of non-lignocellulosic
biomass which has low or no cellulose,
hemicellulose and lignin content. Protein, which is
non-lignocellulosic, is a common important
component of biomass, organic waste, food waste,
animal matter, and algae which are usually wet
biomass. Then, protein gasification characteristics is
important to study that its molecule is different from
carbohydrate and has N-containing. Amino acids
are good representative model compounds of protein
due to they are produced by the hydrolysis of
protein. So far, glycine, alanine, valine, leucine, and
proline have been studied their gasification
characteristics (Samanmulya et al., 2014). These
employed amino acids showed the different
gasification behavior but only the gasification rate of
glycine and alanine are practically identical, and
they were considered to be a standard for
determining amino acid gasification. By the
different stability of the bond between the carboxyl
group and amino group and functional group, the
other three amino acids behave variously from the
standard amino acids and the different sensitivity of
the produced radicals also affect to their gasification
characteristics. Then, more gasification
characteristics data is required to be able to predict
the gasification characteristics of amino acids.
Moreover, the correlation between kinetic rate and
temperature are useful to hypothesize and
understand the reaction mechanism of supercritical
water gasification of biomass (Promdej and
Matsumura, 2011). The reactions between
intermediates were differentiated to be radical and
ionic reactions by their compatibility to Arrhenius
behavior (Yong and Matsumura, 2013). Therefore,
Arrhenius rate law has attracted attention to
determine supercritical water gasification
characteristics of amino acids.
Glutamic acid and arginine are of interest
because they are mostly found in a variety of foods
including animal source and plant source. They are
classified in different category which are acidic and
basic, respectively, and moreover, their gasification
characteristics have not much been studied yet.
This study purpose is to determine the effect of two
carboxyl groups, guanidine functional group, acidity
and alkalinity on supercritical water gasification.
Furthermore, the obtained reaction products were
evaluated based on qualitative and quantitative, and
the kinetics parameters were also elucidated for the
reaction rate of glutamic acid and arginine
gasification.
2 EXPERIMENTAL SECTION
2.1 Experimental Procedure
All gasification experiments were performed using
the tubular flow reactor which was schematically
illustrated in our previous study (Samanmulya et al.,
2014). Briefly, a SS316 steel tube with a length of
12 m and an inner diameter of 2.17mm was used as
the reactor. The reaction temperature was varied
from 500 to 650
o
C (residence times in a range of
86–119 s) and reaction pressure was fixed at 25
MPa. Before the addition of the feedstock, the
reactor pressure was maintained at 25 MPa by
feeding only deionized water and controlled by
back-pressure regulator, and the reactor temperature
was reached the desired temperature. Glutamic acid
and arginine, obtained from Nacalai Tesque with
purity >98%, aqueous solutions of 1.0 wt% were
prepared by dilution in deionized water and fed into
the reactor at a feedstock flow rate of 2 g/min. After
passing through the reactor, the effluent was cooled
down in a heat exchanger, depressurized by a back-
pressure regulator, and then sampled.
2.2 Analytical Methods
The rate of gas generation was measured using a
water displacement method in which we measured
the time required for the effluent gas to fill a vial of
known volume. The gaseous product was
characterized and quantified using gas
chromatography (GC). Carbon dioxide and carbon
monoxide were detected by GC with a thermal
conductivity detector (GC-TCD) using helium as the
carrier gas. Methane, ethene, and ethane were
detected using GC with a flame ionization detector
(GC-FID) using helium as the carrier gas. Hydrogen
was detected by GC-TCD with nitrogen as the
carrier gas.
The liquid product was quantified the amounts of
carbon in the liquid product (non-purgeable organic
carbon, NPOC) and the dissolved carbon gas
product (inorganic carbon, IC) by a total organic
carbon (TOC) analyzer.
Although, gasification of nitrogen contained
molecule that can produce syngas with NOx but N-
containing molecule is finally converted to N
2
via
intermediate compound of NH
3
generation in
supercritical water gasification system and no NOx
is occurred (Goto et al., 1998 and Yakaboylu et al.,
2013).
ICFEPP 2019 - International conference on Future Environment Pollution and Prevention
18

3 RESULTS AND DISCUSSION
3.1 Glutamic Acid Gasification
Based on the previous studies, decomposition
schematic of glutamic acid was proposed as shown
in Figure 1. The schematic has been adapted from
previous work (Samanmulya et al., 2014). The bond
between the carboxyl group and the other parts
(amino group and functional group) is likely to be
cleaved first; considering the instability of the
radicals produced in the following stage, this can be
expected to be the rate determining step for the
gasification. Decomposition of glutamic acid leaves
propionic radical (CH
2
CH
2
COOH) which is rather
unstable. It further decomposes and produces
ethylene and carboxyl radical which are gasified
easily.
Figure 1: The proposed decomposition schematic pathway
of glutamic acids under supercritical water conditions.
Figure 2: Effect of temperature on the product gas
composition of glutamic acid gasification.
The effect of reaction temperature on product gas
composition is shown in Figure 2. A fraction of
ethylene is evident in gas composition, which is
explained by the production of ethylene from
decomposition of propionic radical
(CH
2
CH
2
COOH). Ethylene is further consumed to
produce other gases at later stages, which leads to a
lower ethylene fraction at higher temperatures, while
methane fraction is increased.
3.2 Arginine Gasification
Decomposition of amino acids leads to the
production of radicals (Samanmulya et al., 2014).
Figure 3 shows a schematic of the proposed
decomposition pathways of arginine, the employed
amino acids. This schematic has been adapted from
previous work (Samanmulya et al., 2014). The same
hypothesis as mentioned in glutamic acid
gasification, propyl-guanidine may produce from
arginine decomposition and it is a big molecule that
should stay longer than small radicals. Propyl-
guanidine will further decompose to produce small
molecules and product gas leaving ammonia in
liquid phase. Water gas shift reaction will be
promoted by this alkalinity. The carbon monoxide
reducing is evident in gas composition when the
reaction temperature increased. Moreover, we had
observed the pH of liquid products which were
above 9.
The composition of generated gases from the
arginine gasification as a function of reaction
temperature is shown in Figure 4. Arginine
gasification results in an increased methane fraction
with increasing reaction temperature while carbon
monoxide was decreased. This can be explained as
methanation. At low reaction temperature, we also
observe a carbon monoxide fraction in the product
gas, possibly owing to incomplete gasification.
However, this fraction reduces with increasing
reaction temperature. At 650
o
C, the fraction of
carbon monoxide was reduced while those of carbon
Acidic and Basic Amino Acids Gasification Characteristics under Supercritical Water Conditions
19
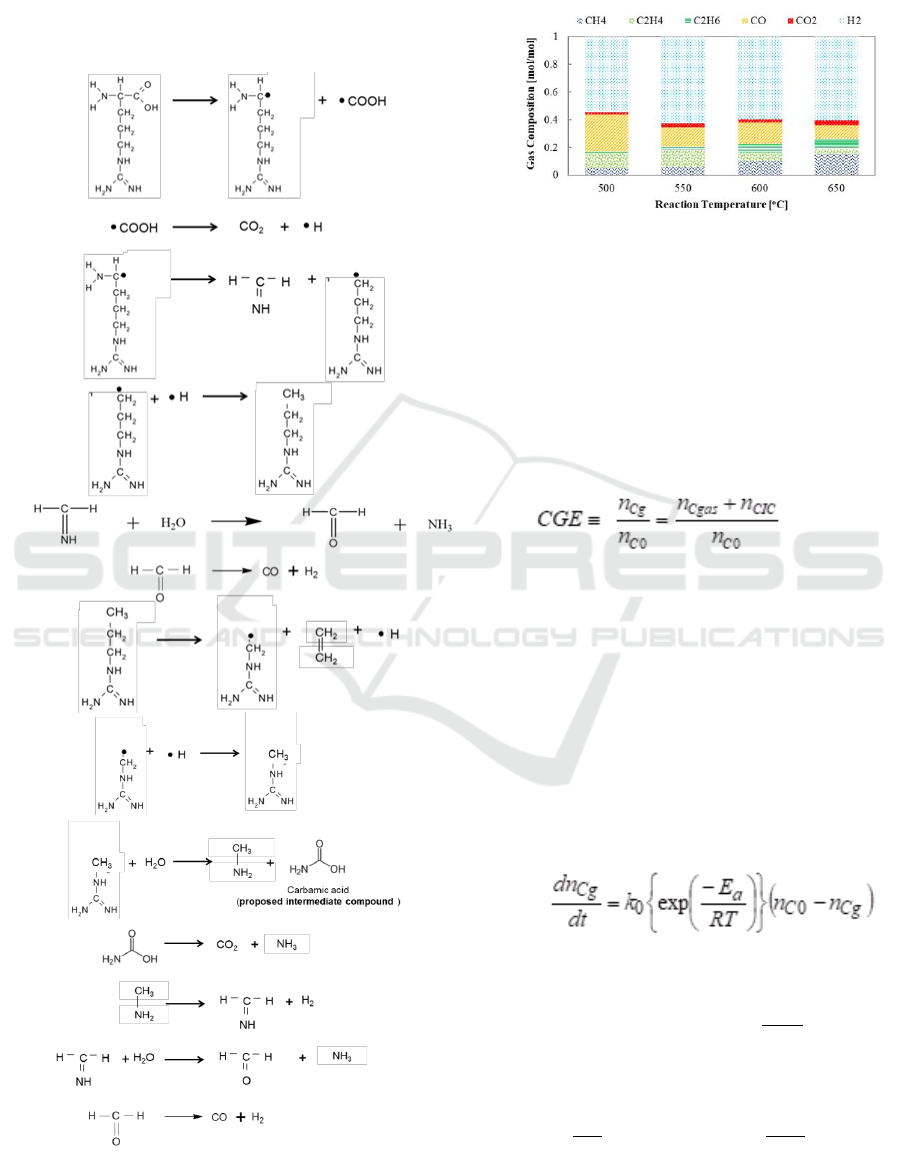
dioxide and hydrogen were increased, owing to the
promotion of the water-gas shift reaction at high
reaction temperature. Ethylene fraction is evident in
product gas composition which supports the
proposed arginine decomposition schematic as
shown in Figure 3.
Figure 3: The proposed decomposition schematic pathway
of arginine under supercritical water conditions.
Figure 4: Effect of temperature on the product gas
composition of arginine gasification.
3.3 Reaction Rate of Glutamic Acid and
Arginine Gasification
Carbon gas product yield or carbon gasification
efficiency (CGE) is defined as the ratio of the
amount of carbon basis in the gas product to that in
the feedstock solution.
(1)
where
0C
n
denote the initial amount of carbon
[mol],
Cg
n
denote the amount of gasified carbon
[mol],
Cgas
n
denote the total amount of carbon in
gaseous product obtained from GC [mol],
CIC
n
denote the total amount of inorganic carbon in liquid
product [mol], and
CGE
denote carbon gasification
efficiency [-].
Assuming the Arrhenius rate law that the
gasification reaction is first order in terms of the
feedstock carbon content, the following equation is
obtained:
(2)
which leads to
t
RT
E
knnn
a
CCgC
expexp
000
(3)
t
RT
E
k
n
n
CGE
a
C
Cg
expexp1
0
0
(4)
ICFEPP 2019 - International conference on Future Environment Pollution and Prevention
20

where
0
k
= pre-exponential factor [s-1],
a
E
=
activation energy [J mol-1],
R
= gas constant [J
mol-1 K-1],
T
= Temperature [K], and
t
= time
[s].
The experimental data of carbon gasification
efficiency was fitted to Eq. (4) and the parameters
were determined.
Figure 5: Gasification characteristics of the employed
amino acids relative to the reported results for glycine and
alanine.
Table 1: Reaction rate parameters of Supercritical Water
Gasification for Glycine, Alanine, Valine, Leucine,
Proline, Glutamic Acid and Arginine
Amino
acids
Pre-
exponential
factor [s
-1
]
Activation
Energy
[kJ/mol]
Glycine and
Alanine
7.37 ×10
5
131
Valine
6.97 ×10
1
70
Leucine
7.37 ×10
5
135
Proline
1.96 ×10
2
73
Glutamic
Acid
4.50 ×10
2
77
Arginine
2.50 ×10
2
75
Figure 5 shows the effect of reaction temperature
on carbon gasification efficiency of the employed
amino acids relative to the reported results for
glycine and alanine. Note that the gasification
characteristics of glycine and alanine are identical
and they are shown by a solid black line. The
gasification efficiency increased with reaction
temperature as was observed for glucose (Xu et al.,
1996), which implies the Arrhenius behavior. The
gasification efficiency of the employed amino acids
is consistent with Arrhenius behavior indicating by
the red dashed and pink dotted line in the figure.
The reaction parameters were pre-exponential factor
and activation energy which are shown in Table 1
including with those of glycine, alanine, valine,
leucine and proline for comparison purpose. The
fitting results using these parameters are also shown
in Figure 5. Gasification rate of arginine is slower
than that of glycine and alanine although the
activation energy is also lower. The calculated
results and experimental data are in a good
agreement. Experimental results and theoretical
results using the Arrhenius parameters from Table 1
showed good correlation.
4 CONCLUSION
The supercritical water gasification of glutamic acid
and arginine can be characterized by first order
kinetics with the Arrhenius equation rate constant,
and the reaction parameters were determined. The
gasification rate of the two selected amino acids
were lower than that of glycine and alanine, even the
activation energy is lower that of glycine and
alanine. Glutamic decomposition produces
propionic acid radical and it further decomposes and
produces ethylene and carboxyl radical which are
easily to gasify. Arginine decomposition leads to
produce propyl-guanidine and it will further
decompose to produce small compounds and
product gas, especially for ethylene that has evident
in product gas composition.
ACKNOWLEDGMENT
This study was supported by Thermal Engineering
Laboratory, Hiroshima University.
REFERENCES
Antal, Jr. M. J., Allen, S. G., Schulman, D., Xu, X.,
2000. Ind. Eng. Chem. Res. 39, 4040.
Goto, M., Nada, T., Ogata, A., Kodama, A., Hirose,
T., 1998. J. Supercrit. Fluids 13, 277.
Matsumura, Y., Hara, S., Kaminaka, K., Yamashita,
Y., Yoshida, T., Inoue, S., Kawai, Y., Minowa,
T., Noguchi, T., Shimizu, Y., 2013. J. Jpn.
Petrol. Inst. 56, 1.
Promdej, C., Matsumura, Y., 2011. Ind. Eng. Chem.
Res. 50, 8492.
Samanmulya, T., Inoue, S., Inoue, T., Kawai, Y.,
Kubota, H., Munetsuna, H., Noguchi, T.,
Acidic and Basic Amino Acids Gasification Characteristics under Supercritical Water Conditions
21

Matsumura, Y., 2014. J Jpn. Inst. Energy 93,
936.
Xu, X., Matsumura, Y., Stenberg, J., Antal, Jr. M. J.,
1996. Ind. Eng. Chem. Res. 35, 2522.
Yakaboylu, O., Harinck, J., Smit, K. G. G., Jong,
W.D., 2013. Biomass Bioenergy 59, 253.
Yong, T. L. K., Matsumura, Y., 2013. Ind. Eng.
Chem. Res. 52, 9048.
ICFEPP 2019 - International conference on Future Environment Pollution and Prevention
22
