
Synthesis Succinic Galactomannan from Galactomannan Arenga
pinnata Merr. and Succinic Anhydride using Microwave Method
Juliati Br. Tarigan*, Mutiara H. Siregar
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Jl. Bioteknologi No.
1 Kampus USU, Medan, Indonesia
Keywords: Arenga pinnata Merr., endosperm, Galactomannan, Succinic Galactomannan, Microwave.
Abstract: The synthesis succinic galactomannan has been conducted through esterification reaction of galactomannan
Arenga pinnata and succinic anhydride using sodium bicarbonate as catalyst under the radiation of
microwave. The experiment divided by two steps which first step was the extraction of galactomannan from
Arenga pinnata rendering yield of 4.20% and have confirmed by spectroscopy Fourier Transform Infrared
(FT-IR). The second step was esterification with succinic anhydride using NaHCO3 as catalyst under the
radiation of microwave for 3, 5, 7, 9 and 11 min. Spectroscopy FT-IR showed the stretching vibration of the
carbonyl group (C=O) of the ester at a wavelength of 1735 cm-1 which established the formation of succinic
galactomannan. The substitution degree of succinic galactomannan determined by titration method obtained
the maximum value at 1.527 for 9 min radiation time. The scanning electron microscopy (SEM) images
showed that succinic galactomannan has rough surface morphology than galactomannan Arenga pinnata.
1 INTRODUCTION
Galactomannan is a polysaccharide which found
abundant and are specifically produced from beans.
One source of galactomannan in Indonesia is
“kolang – kaling” or Arenga pinnata endosperm
(APE). APE is a semi-mature, soft, chewy and
slightly clear white sugar palm endosperm which is
obtained after going through the processing process
(Kooiman, 1971). Galactomannan consists of two
types of sugar monomer units, namely mannose and
galactose. Mannose is the main component and
galactose is a minor component. The number of
galactose units in the polysaccharide is always
smaller than that of mannose (Mathur, 2011).
Comparison of galactose and mannose will affect the
properties of the galactomannan. The advantages of
galactomannan are the chain of galactose branches
which is polar and mannose (straight chains) is
nonpolar. The molecular weights of galactomannan
from APE are diverse and vary from 6000 to 17000
(Kooiman, 1971). Galactomannan has a structure (1-
4) -β-D-mannopyranose as a straight chain and unit
(1-6) -α-D-galactopyranose as its branch (Dey,
1978). Galactomannan has been extracted from
sugar palm seeds using ethanol and water followed
by centrifugation to separate the precipitate.
Galactomannan content was obtained at 4.58% and
is feasible to eat (Tarigan, 2014). To improve
galactomannan properties can be done by modifying
the compound using chemical reagents. One way is
to convert galactomannan to its ester form.
Esterification reactions of polysaccharides are
very common and have been used to modify and
increase the functional properties of derivate
polysaccharide. Succinic esters of Arabic gum have
been used as stabilizers of emulsions and used in
microencapsulation processes (Sarkar and Singhal,
2011). Dong and Tian (1999) have been successfully
synthesized palmitic ester of guar galactomannan
and used as emulsion stabilizers. Prashanth et al.
(2006) have synthesized galactomannan esters using
succinic anhydride, acetic anhydride and n-octenyl
succinate anhydride using catalyst sodium
bicarbonate at a temperature of 28˚C, 40˚C, 60˚C for
2 hours. Sarkar and Singhal (2011) demonstrated the
synthesis of guar gum esters with n-octenyl
succinate anhydrous using sodium bicarbonate as a
catalyst at a temperature of 60˚C, 75˚C, 90˚C for 3
hours. Previous researchers have modified
galactomannan compounds through acetylation
using acetic anhydride using sulfuric acid catalysts
under reflux conditions at 50˚C for 6 hours. In
Tarigan, J. and Siregar, M.
Synthesis Succinic Galactomannan from Galactomannan Arenga pinnata Merr. and Succinic Anhydride using Microwave Method.
DOI: 10.5220/0008854200690073
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 69-73
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
69

general, the reaction of polysaccharide ester
production is carried out prolonged reaction time.
Therefore it is necessary to find out a novel method
to produce polysaccharide ester in short reaction
time. Microwave has been known could enhance
organic reaction and shorten reaction time producing
a high yield of product (Mohd Fuad et al., 2019).
Based on that, the aim of this study was to
synthesis succinic galactomannan from the reaction
of galactomannan with succinic anhydride using
sodium bicarbonate as a catalyst under microwave
irradiation. The succinic galactomannan esters
obtained were analyzed for functional group changes
by FT-IR spectrophotometer, SEM and the degree of
substitution by titration.
2 MATERIALS AND METHODS
2.1 Materials
“Kolang-kaling” was bought from a local traditional
market in Medan – Indonesia. All chemicals used in
this study were purchased from local chemical
dealers and have been used without any purification.
2.2 Methods
2.2.1 Extraction Galactomannan from APE
The APE which has been cleaned and chopped as
much as 100 grams is mashed with a blender by
adding 1000 mL of distilled water and stored in the
refrigerator for 24 hours. Then, it was centrifuged at
an average speed of 8500 rpm for 50 minutes.
Ethanol is added to the supernatant with a volume
ratio of 1: 2 and stored in a refrigerator for 24 hours.
The precipitate formed is then soaked using ethanol
and dried in a desiccator.
2.2.2 Synthesis Succinic Galactomannan
One gram of galactomannan was added with 0.5
gram of sodium bicarbonate and 2 mL of ethanol.
Next, 2 g of succinic anhydride was added followed
by stirring for 20 minutes. Then the mixture is
heated in the microwave for 3 minutes. After that,
the mixture was added with 10 mL ethanol 50% and
then followed by neutralization using NaOH 2N.
The precipitate was washed with 75% ethanol and
then washed with ethanol. The precipitate was dried
in a desiccator (Prashanth et al., 2006).
2.2.3 Determination of Substitution Degree
One gram of succinic galactomannan was put in
Erlenmeyer and then added with 10 mL of water
containing 5 mL NaOH 0.5M followed by stirring
for 30 minutes at room temperature. The solution
then titrated with HCl 1N using phenolphthalein
indicators (Sarkar and Singhal, 2011). The value of
the degree of substitution can be calculated based on
the equation below.
(1)
(2)
Where W is substitution of succinic acid, V
blank
is
the HCl volume for the blank solution and V
sample
is
the HCl volume required for neutralizing the sample.
N is normality of HCl, M is molecular weight of
succinic acid.
3 RESULTS AND DISCUSSIONS
3.1 Extraction of Galactomannan from
APE
About 300 grams of APE was extracted using
distilled water and then centrifuged at a speed of
8500 rpm for 50 minutes and then the supernatant
was added ethanol 96% to formed precipitate which
was washed using ethanol. The galactomannan
extracted was 12.6205 grams (4.20%) which were
then characterized by FT-IR.
3.2 FT-IR analysis
The galactomannan obtained was analyzed using
FT-IR to determine the presence of functional
groups. The FT-IR results obtained are shown in
Figure 1.
Figure 1: F-IR spectra of galactomannan
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
70
The FT-IR spectrum of galactomannan showed
vibration peaks in the wavenumber area of 3410 cm
-
1
represent a stretching vibration of the -OH group
from polysaccharides. This is supported by
absorption bands at wave number 1635 cm
-1
which
showed vibrations –OH bending from water
absorption (Gong et al., 2012). The absorption band
at wave number 2924 cm
-1
shows the stretching of -
CH vibration, which is supported by the presence of
absorption bands at wave number 1381 cm
-1
represent the bending –CH vibrations (Singh et al.,
2009). The 871 cm
-1
band shows the characteristics
of β-D-mannopyranose bond in the polysaccharide
and in the 810 cm
-1
band shows the characteristics of
α-D-galactopyrannose bonds (Buriti et al., 2014).
3.3 Synthesis and Characterization
Succinic Galactomannan
The result obtained from the esterification
galactomannan with succinic acid anhydride is white
solids. The yields of succinic galactomannan are
shown in Table 1.
Table 1: The yield of succinic galactomannan
Reaction
Time
(min)
Weight of
Galactomannan
(g)
Weight of
Succinic
Galactomannan
(g)
3
1.0608
1.2884
5
1.0065
1.1765
7
1.0870
1.0289
9
1.0089
1.0023
11
1.0054
0.9473
The galactomannan reacted with succinic
anhydride produces succinic galactomannan which
has different weights. The decrease in weight of
succinic galactomannan produced was due to the
increasing reaction temperature during heating
which caused degradation of the polysaccharide
chain in the galactomannan. The degradation of the
polysaccharide chain causes a decrease in molecular
weight produced rendering increasing solubility
which during washing it dissolves. Prashanth et al.
(2006) stated that the molecular weight of gum
acetate (~1500 kDa with HPSEC) decreases when
the reaction takes place at a temperature of 60
o
C
compared to gum and other esters which are reacted
at low temperatures (~2000 kDa). Succinic
galactomannan from various reaction time obtained
was analyzed using FT-IR to determine the changes
in functional groups. The FT-IR results obtained are
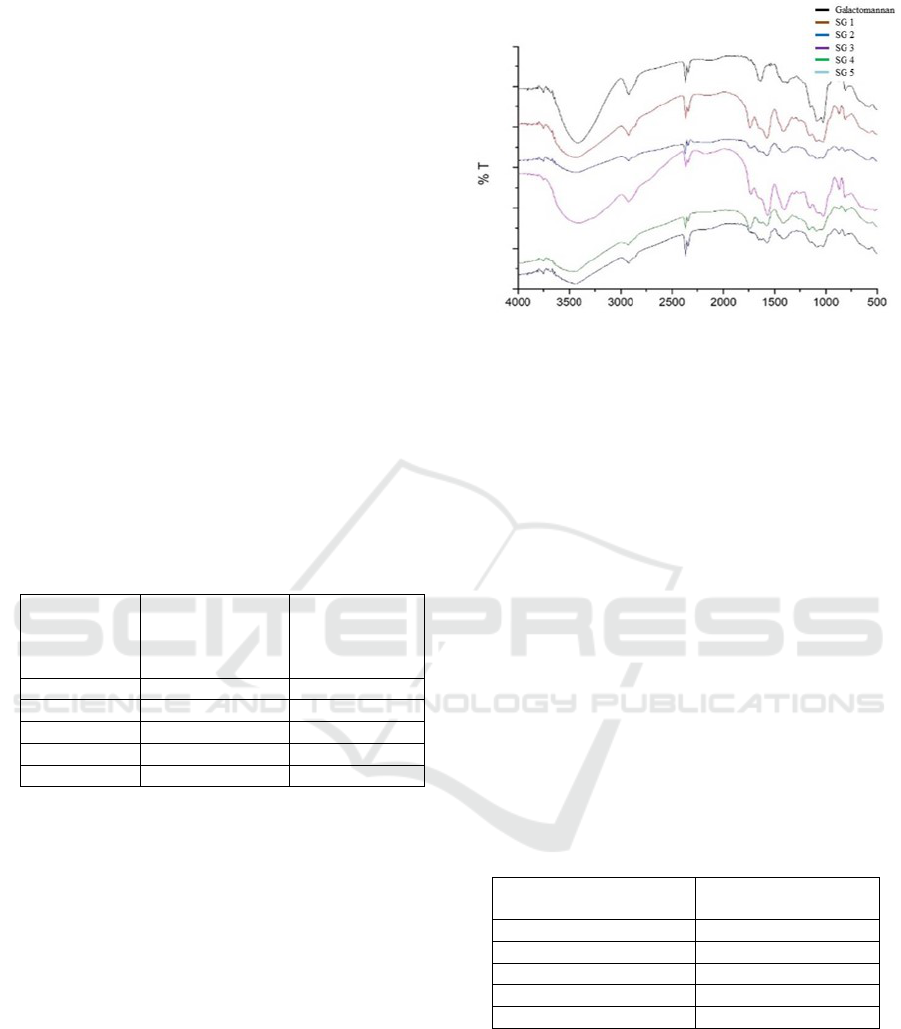
shown in the following Figure 2.
Figure 2: FT-IR spectra of galactomannan and succinic
galactomannan in various time reaction
FT-IR was used to confirm the formation of succinic
galactomannan. The FT-IR spectrum showed the
absorption band appears at wavenumbers of 1730 -
1750 cm
-1
which is represent the stretching vibration
of C=O which and it is supported by absorption
bands at wave number of 1379 cm
-1
which shows the
CH bending vibration and the vibration stretching of
CO in the group of -O-(C=O)-CH3 at wavelength of
1245 - 1250 cm
-1
. The changes of wavelength from
1024 to 1031 cm
-1
shows the vibration stretching of
C-O-C on galactomannan to galactomannan ester
(Prashanth et al., 2006).
3.4 The Substitution Degree
The substitution degrees of succinic galactomannan
determined by titration method are presented in
Table 2.
Table 2: The substitution degree of succinic
galactomannan in various reaction time
Reaction time
(min)
Substitution degree
value
3
0.404
5
0.460
7
0.503
9
1.527
11
1.090
As can be seen from Table 2, the substitution degree
was increased in the increasing of reaction time in
the microwave with the highest of substitution
degree values occurred at 9 minutes is 1.527.
However, the substitution degree value was
decreased at 11 minutes showed of 1.09. This is
probably due to the temperature that was too high in
the microwave at reaction time of 11 minutes which
caused the degradation of the galactomannan
Synthesis Succinic Galactomannan from Galactomannan Arenga pinnata Merr. and Succinic Anhydride using Microwave Method
71

indicated by the product was blackish brown. Based
on that observations, the longer the reaction time in
the microwave, the temperature increases and thus if
the temperature increases, the value of the
substitution degree is higher. Prashanth et al. (2006)
also showed that increasing in temperature could
increase in the substitution degree value.
3.5 SEM Analysis
The SEM images of succinic galactomannan
produced from 9 minutes reaction time is presented
in Figure 3. As can be seen in Figure 3,
galactomannan and succinic galactomannan showed
changes in surface morphology. The galactomannan
showed a smooth surface shape that is irregular and
fused to one another, whereas succinic
galactomannan showed that the surface is a bit rough
and bumpy indicates an esterification reaction on
galactomannan has been occurred.
Figure 3: The SEM images of (A) galactomannan and (B)
succinic galactomannan
Figure 4: The SEM images polysaccharide of (A) hard
APE, (B) soft APE and carboxymethyl
polysaccharide from (C) hard APE, (D) soft
APE
4 CONCLUSIONS
The yield of galactomannan extracted from APE
using ethanol solvent was 12.6205 grams. Succinic
galactomannan has been synthesized through a
reaction between galactomannan and succinic acid
anhydride with NaHCO
3
as a catalyst under
microwave irradiation for several reaction times.
The FT-IR spectra confirm the formation of
galactomannan ester by appearance a spectrum at a
wavelength of 1735 cm
-1
indicating the vibration of
C=O of the ester compound. The highest degree of
substitution obtained was 1.527 which occurred
from reaction time of 9 minutes. The SEM images
showed the changes in the morphology of
galactomannan which were smooth and fused to one
another and rough and bumpy on succinic
galactomannan.
REFERENCES
Buriti, F. C. A., Dos Santos, K. M. O., Sombra, V. G.,
Maciel, J. S., Teixeira Sá, D. M. A., Salles, H. O.,
Oliveira, G., De Paula, R. C. M., Feitosa, J. P. A.,
Monteiro Moreira, A. C. O., Moreira, R. A. & Egito,
A. S. 2014. Characterisation of partially hydrolysed
galactomannan from Caesalpinia pulcherrima seeds as
a potential dietary fibre. Food Hydrocolloids, 35, 512-
521.
Dey, P. M. 1978. Biochemistry of Plant Galactomannans.
Advances in Carbohydrate Chemistry and
Biochemistry.
Dong, C. & Tian, B. 1999. Studies on preparation and
emulsifying properties of guar galactomannan ester of
palmitic acid. Journal of Applied Polymer Science, 72,
639-645.
Gong, H., Liu, M., Chen, J., Han, F., Gao, C. & Zhang, B.
2012. Synthesis and characterization of carboxymethyl
guar gum and rheological properties of its solutions.
Carbohydrate Polymers, 88, 1015-1022.
Kooiman, P. 1971. Structures of the galactomannans from
seeds of Annona muricata, Arenga saccharifera, Cocos
nucifera, Convolvulus tricolor, and Sophora japonica.
Carbohydrate Research, 20, 329-337.
Mathur, N. 2011. Industrial galactomannan
polysaccharides, CRC Press.
Mohd Fuad, M. a. H., Hasan, M. F. & Ani, F. N. 2019.
Microwave torrefaction for viable fuel production: A
review on theory, affecting factors, potential and
challenges. Fuel, 253, 512-526.
Prashanth, M. R. S., Parvathy, K. S., Susheelamma, N. S.,
Harish Prashanth, K. V., Tharanathan, R. N., Cha, A.
& Anilkumar, G. 2006. Galactomannan esters—A
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
72

simple, cost-effective method of preparation and
characterization. Food Hydrocolloids, 20, 1198-1205.
Sarkar, S. & Singhal, R. S. 2011. Esterification of guar
gum hydrolysate and gum Arabic with n-octenyl
succinic anhydride and oleic acid and its evaluation as
wall material in microencapsulation. Carbohydrate
Polymers, 86, 1723-1731.
Singh, V., Srivastava, A. & Tiwari, A. 2009. Structural
elucidation, modification and characterization of seed
gum from Cassia javahikai seeds: A non-traditional
source of industrial gums. International Journal of
Biological Macromolecules, 45, 293-297.
Tarigan, J. B. 2014. Karakterisasi Edible Film Yang
Bersifat Antioksidan Dan Antimikroba Dari
Galaktomanan Biji Aren (Arenga pinnata) Yang
Diinkorporasi Dengan Minyak Atsiri Daun Kemangi
(Ocimum basilicum L.). Doktor Disertasi, Universitas
Sumatera Utara.
Synthesis Succinic Galactomannan from Galactomannan Arenga pinnata Merr. and Succinic Anhydride using Microwave Method
73
