
Synthesis and Identification of Furfural from Cocoa Pod Husk
(CPH) with Pretreatment Process before Hydrolysis Process
Lisa Aulia Lubis, Amir Husnin, Maulida
Department of Chemical Engineering, Universitas Sumatera Utara, Jl. Almamater Kampus USU, Medan, North Sumatera,
Indonesia
Keywords: Biorefinery, Cocoa Pod Husk, Furfural, Pretreatment, Process Optimization
Abstract : Furfural is an organic compound that can be produced from agricultural waste such as oats, corn cobs, rice
husks, bagasse, and sawdust. Cocoa pod husk is a renewable raw material for furfural manufacturing.
Furfural synthesis from cocoa pod husk is an attempt to create value-added of cocoa pod husk. Furfural
synthesis was based on the hydrolysis of pentosan into xylose which was then dehydrated to furfural. Cocoa
pod husk waste contains pectin and lignin which can interfere hydolysis process. It make use pretreatment
process to reduce pectin and lignin. Percentages pectin, lignin and pentosan before pretreatment
were 9.2 ± 0.5,
14.7% and 38.9% and after pretreatment to be 1.7 ± 0.01, 4.13% and 37.5%. In this study
used hydrolysis temperature variations (110, 120, 130 and 140)
0
C and hydrolysis time (10, 20, 30, 40 and
50) minutes. The optimum conditions obtained at temperature and time of hydrolysis of 130
0
C and 30
minutes, weight furfural obtained was 6.728 g/7,50g pentosan or 6,728g/20g of cocoa pod husk and yield
furfural obtained was 82.2%. This shows that cocoa pod husk has a high potential to be converted into
furfural and can be used as a renewable raw material in furfural manufacturing. Furfural identified by color
test using aniline acetate 1:1, Gas Cromatographic Mass Spectrometry (GCMS) and infra-
spectrophotometer (FTIR).
1 INTRODUCTION
In the last decade there has been climate change and
depletion of fossil fuels which were the main
reasons humans use renewable raw materials such as
lignocellulose residues to produce fuel. In recent
years many researchers have devoted themselves to
the exploration and development of efficient
production for furfural.Many studies using
renewable raw materials for furfural production are
one of them biomass agricultural wastes (Liu, 2018).
Raw material from biomass has several
advantages, They are biomass as renewable source,
hemicellulose in biomass is high, furfural production
can be produced more than 98%, besides it was easy
to obtain, low cost and it was not pollute
environment (Machad, 2016).
Cocoa is one of the plants that produce biomass
of agricultural waste. Cocoa (Theobroma cacao L) is
the nameof fruit fromcocoa tree. Cocoa fruit consists
of seeds and shell or test. The biggest waste
component in cocoa is cocoa pod husk (CPH). The
cocoa pod husk are 70% -75% of the total cocoa
fruit (Daud, 2014). The high production made from
cocoa beans increases the world's cocoa pod husk
waste in almost 700,000 tons in year (Okiyama,
2017).
The reason of this study using cocoa pod husk as
a raw material for produce furfural is renewable,
high amount of hemicellulose, increase demand for
chemicals produce from agricultural waste, and the
growing awareness of the environment. It has
potential as a raw material for produce furfural. The
composition of CPH can be show in Table 1.
Table 1: Chemical Composition of Cocoa Pod Husk
(CPH)
Komponen
* Percentage
(%)
**(%) *** (%)
Cellulose 28.78 35.40 44.69
Hemicellulose 8.70 37.00 11.15
Lignin 42.90 14.70 34.82
Ash 1.87 12.30 7.40
Sumber : *(Syam, 2000)
**(Daud, 2014)
***(Nazir, 2016)
160
Lubis, L., Husin, A. and Maulida, .
Synthesis and Identification of Furfural from Cocoa Pod Husk (CPH) with Pretreatment Process before Hydrolysis Process.
DOI: 10.5220/0008863801600164
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 160-164
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
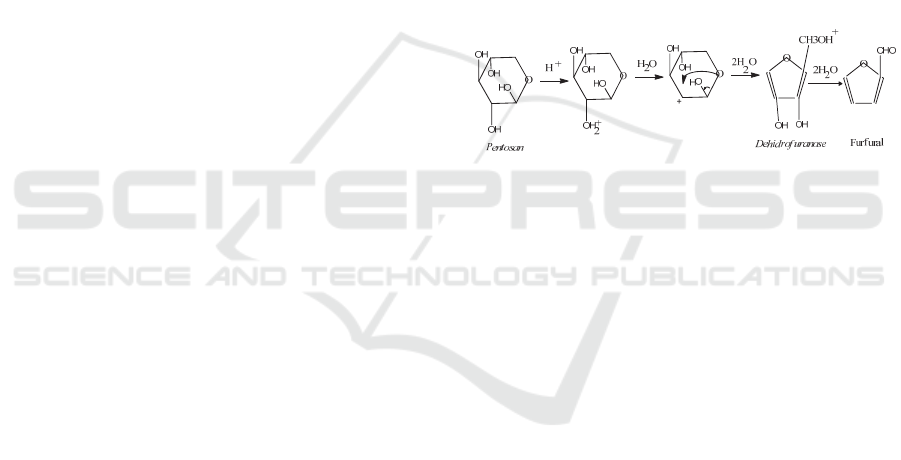
There were three step to produce furfural. First
step waspetreatment, second step was hydrolysis
process from pentoseto xylose and last step was
dehydrated xylose to furfural. The stoichiometric
equation for reaction is as below (Branca, 2012):
C
5
H
10
O
5
→ C
5
H
4
O
2
+ 3H
2
O (1)
Biomass as raw materials in the process of
produce chemicals was often carried out
pretreatment process to reduce the presence of
compounds such as lignin, pectin and other
compounds it can disturb hydrolysis process,
pretreatment was done first. In study conducted by
(Mao, 2012) using raw material corn cobs the
pretreatment process was not carried out, so the
maximum yield was produced only 67.89% due to
the presence of other compounds that inhibited the
hydrolysis process, while the study was conducted
(Liu, 2018) using a pretreatment process produces a
maximum yield of 73%. It shown the pretreatment
process can increase yield furfural.
In this study used a renewable raw materials. It
was cocoa pod husk with pretreatment process for
removal of pectin and lignin. Cocoa pod husk was a
renewable raw material, therefor researchers also do
variation of temperature and reaction time in the
hydrolysis process, to find out the optimum
conditions and the highest furfural yield that can be
produced by cocoa pod husk.
2 MATERIALS AND METHODS
2.1 Raw Material
The raw material for cocoa pod husk was obtained
from cocoa fruit trees in Naga Timbul Village,
Indonesia.
The cocoa pod husk from farm was collected,
washed, dried at 110
0
C until the water content is ±
10%, after dried CPH was cut into sizes up to 1cm
(Nazir, 2016).
2.2 Process Pretreatment
2.2.1 Pectin Extraction
Extraction of pectin done for elimination of pectin in
cocoa pod husk. It used a citric acid. 100g of sample
were added 1:25 citric acid solvent, extraction time
of 3 hours, pH 2.5 at 95
o
C (Nazir, 2016).
2.2.2 Reduction of Lignin
Samples from the pectin extraction process added
4% NaOH solution with a solid-liquid ratio of 1:25
was autoclaved at 121
o
C for 100 minutes, filtered
and washed until netral pH, dried at 105
o
C for 6
hours (Nazir, 2016).
2.3 Furfural Production
As 20g of sample was added H
2
SO
4
3M (Stein,
2011) with a ratio of 1:15 (Kaur, 2011), added NaCl
20g reacted to batch reactor with variationsreaction
temperature (110, 120, 130 and 140)
o
C (Peleteir,
2016) and reaction time (10, 20, 30, 40 and 50)
minutes. Hydrolysis and dehydration results of vapor
phase-shaped samples contained in furfural
compounds. The reactions that occur can be show in
Figure 1 (Branca, 2012).
Figure 1: Hydrolysis reaction of Pentosan to Xylose and
Dehydration to Furfural (Branca, 2012)
2.4 Analysis
Analysis of the composition of cellulosa,
hemicellulose and lignin with chesson methode and
pentosan in cocoa pod husk before and after
pretreatment was carried out by gravimetri (Griffin,
1972). Furfural analysis obtained by color test of
aniline acetate 1:1, GC-2010 Serial No.
020504702444 Shimidzu Corp) and FTIR Boil
SHIMIDZU at laboratorium. Department Chemical
Engineering. Lhoksumawe State Polytechnic.
3 RESULTS AND DISCUSSION
3.1 Raw Material Composition
The composition of lignin, cellulose, hemicellulose,
pentose and pectin, before and after pretreatment
show in Table 2: In these results it show that the
composition of lignin was reduced to 10.57% after
the pretreatment process. Pentosan also reduction of
1.4%. This is because a little pentosan bond also
reacts.
Synthesis and Identification of Furfural from Cocoa Pod Husk (CPH) with Pretreatment Process before Hydrolysis Process
161
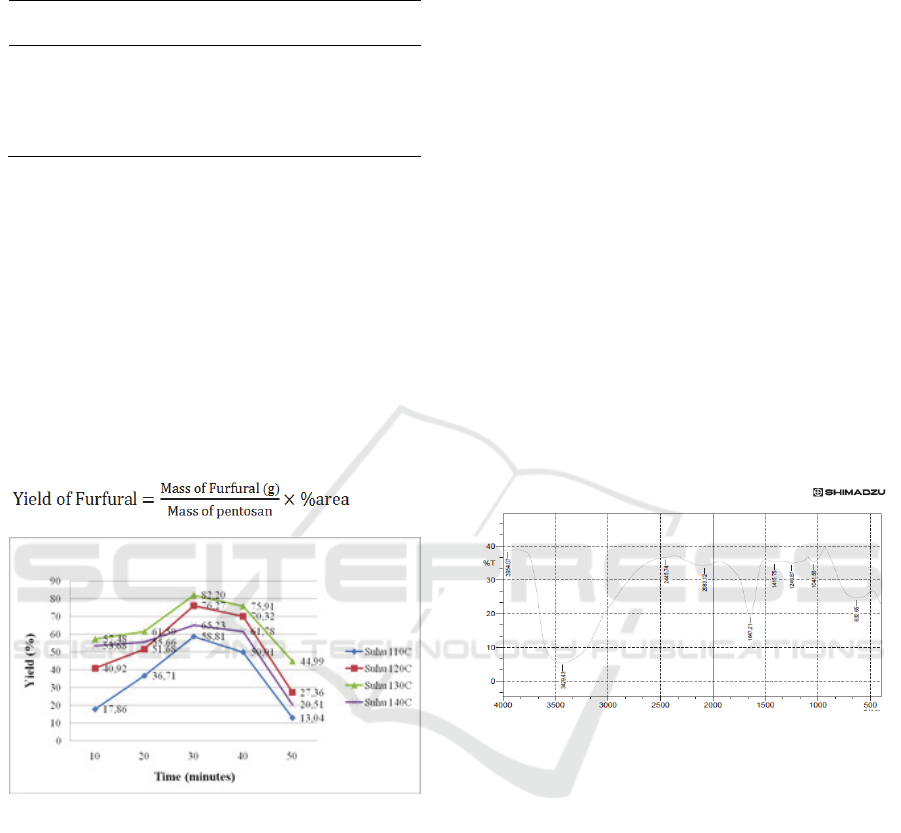
Table 2: Results of Analysis Composition Cocoa Pod
Husk
Component
Before
Pretreatmen
t
After
Pretreatmen
t
Lignin 14.81% 4.13%
Hemicellulose 42.10% 40.30%
- Pentosan 38.91% 37.50%
Cellulose 40.00% 37.31%
Pectin 9.2 ± 0.5 1.7 ± 0.01
Result of composition CPH in this study was
different with other researcher. It was show in Tabel
1. This is due to differences in nutrients in the soil in
each region.
3.2 Effect of Time and Temperature to
Yield of Furfural.
In the process of produced furfural reaction time and
temperature greatly affect furfural yield. The effect
of the reaction time and temperature on furfural
yield show in Figure 2.
(1)
(1)
Figure 2: Effect of Time and Temperature to Yield of
Furfural.
The results of this study indicate the highest
yield value at 130
o
C with a time of 30 minutes of
82.20%. From these data it show at the reaction time
of 10 to 30 minutes the resulting furfural yield
increases, while at the reaction time of 40-50
minutes the resulting furfural yield decreases. This
was because the longer reaction was carried out, the
more side products are formed and can degrade
furfural (Peleteiro, 2016). The by-products formed
are 2-Furancarboxaldehyde, 5-methyl, the side
product data obtained from the results of analysis
with Gas Cromatographic Mass Spectrometry
(GCMS) conducted by researchers. Furfural yield
also increased from 110
o
C - 130
o
C and decreased at
140
o
C. Further demonstrating that thereaction
selectivity can be conveniently tuned by adjusting
thetemperature of the reaction. Reversely, an
increase of the reaction temperature to 140
o
C
lowered the yield of furfural, mostly due to its
degradation or condensation (Liu, 2014).
3.3 Analysis of Aniline Acetate
In this study the color test of the product by using
aniline acetate (1:1) to identify the presence or
absence of furfural compounds in the products. The
results of this color test showed a positive presence
of furfural compounds in the product of hydrolysis,
color was change to be red when aniline acetate
added to product.
3.4 Analysis with FTIR and GCMS
After testing with aniline acetate which stated the
presence of furfural, furfural test was carried out
using Fourier Transform Infra Red (FTIR) and
GCMS. FTIR test can be show in Figure 3.
Figure 3: Furfural Results by Using FTIR Simidzu.
The IR spectrum (Figure: 3) shows a very strong
absorptionC = O (1600 - 1700 cm
-1
). sample
obtained a very strong wave number absorption peak
that is equal to 1647.21 cm
-1
. This absorption shows
a very significant functional group (C = O). Internal
hydrogen bonding which occurs in conjugated
unsaturated aldehydes (Ambalkar et al, 2017).
Absorption peak of 2445.74 cm
-1
approaching
the presence of C-H aldehyde (2800 - 2860cm
-1
).
The presence of an aromatic C = C bond is shown by
the appearance of stretching vibrations C = C
aromatic (1475 - 1600cm
-1
) in the area of about
1415.75cm
-1
nearing the presence of the cluster. The
broad peaks observed at vibrations of 3400 to 2400
cm
-1
from the sample showed wave absorption peaks
of 3429.43 cm
-1
and 2445.74 cm
-1
, which indicated
that the aldehyde bond of the absorption complex
showed aldehyde stretching. If the sample has an
ester - O - peak, C = C peak will be observed at
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
162

1685 cm
-1
to 1660 cm
-1
(Ong, 2007), but the sample
does not show the same absorption. can be show in
Table 3.
Table 3: Furfural Vibration
No
.
Vibration Furfural
Standard
1. Streching aldehyde complex 3429.43
2. Stretching C-H aldehyde 2445.74
3. Stretching C=O aldehyde 1647.21
4. Stretching C=C aromatic 1415.75
5. Stretching C- aldehyde 1249.87
6. Stretching C-O-C 1041.56
Based on the furfural standard vibration value it
can be concluded that the compound produced from
the hydrolysis of cocoa pod huskwas furfural
because it shows spectra which are almost identical
to the standard furfural vibration. Based on the
furfural standard vibration value it can be concluded
that the compound produced from the hydrolysis of
cocoa fruit skin is furfural because it shows spectra
that are identificationof furfural. Further furfural can
be identified with GCMS. The results of GCMS
identification can be show in Figure 3.
Figure 3: Furfural Results using GCMS Simidzu.
The analysis use GC-2010 Serial No.
020504702444 Shimidzu Corp) strengthens that
hydrolysis compounds are furfural. The furfural
compound for the process with pretreatment was
shown at peak 3, retention time 7,629, area
119881925% area 92.65%.
4 CONCLUSIONS
The conclusion of this study is furfural can be
produced using renewable raw materials. It is cocoa
pod husk. The pretreatment process can reduce
lignin until 10.57% and pentosan until 1.4%.
Pentosan and lignin found in cocoa pod husk after
pretreatment process was 4.13% and 37.5%. The
optimum conditions obtained in the hydrolysis
process of cocoa pod husk with a pretreatment
process and dehydration process attemperature
130
o
C and reaction time of 30 minutes produced a
yield of 82.02% obtained from 6.725g / 7.05g
pentose.
REFERENCES
Ambalkar, V. U and Talib, M., 2017. Synthesis and
Identification of Furfural from Sunflower
Husk.International Advanced Research Journal
in Science, Engineering and Technology. Vol 4
Branca, C., Blasi, C. D. and Galgano, A.,
2012.,Catalyst Screening for the Production of
Furfural from Corncob Pyrolysis. Energy and
Fuels. 26: 1520-1530
Daud, Z., Awang, H., Kassim, A.S.M, Hatta,
M.Z.M. and Aripin, A.S., 2014. Cocoa Pod Husk
and Corn Stalk:Alternative Paper Fibres Study
on Chemical Characterization and
Morphological Structures.Advanced Materials
Research. 911: 331-335.
Griffin., Castle, R., Little, A.D., 1972. Technical
Methods of Analysis. McGraw-Hill Book. 298
Kaur, Ramandeep., 2011.,Hemicellulosic Furfural
Production from Sugarcane Bagasse Using
Different Acids.Sugar Tech. 13: 166-169.
Liu, F., Boissou, F., Vignault, A., Lemee, L.,
Marinkovic, L., Estrine, B., Vigier, K. D. O. And
Jerome, F.Conversion of Wheat Straw to
Furfural and Levulinic Acid in a Concentrated
Aqueous Solution of Betaıne
Hydrochloride.Royal Society of Chemistry
(RSC) Adv. 4, 28836.
Liu, L., Chang, H., Jameel, H. and Park, S., 2018.
Furfural production from a Pre-hydrolysate
Generated using Aspen and Maple Chip.Biomass
and Bioenergy. 104: 1-16
Machado, G., Leon, S., Santos, F., Lourega , R.,
Dullius, J., Mollmann, M.E. and Eichler, P.,
2015. Literature Review on Furfural Production
from Lignocellulosic Biomass.Natural Resources.
7: 115-129.
Nazir, N., Novelina., Juita, E., Amelia, C. And Fatli,
R., 2016. Optimization of Pretreatment Process
of Cocoa Pod Husk Using Various Chemical
Solvents. Advanced Science Engineering
Information Technology. 6: 2088-5334
Synthesis and Identification of Furfural from Cocoa Pod Husk (CPH) with Pretreatment Process before Hydrolysis Process
163

Ong, H.K and Sashikala, M., 2007.Indentification of
furfural synthesized from pentosan in rice husk.
J.Trop. Agric. Nd Fd. Sc. Vol 35, 2007:305-312.
Okiyama, D.C.G., Navarro, S. L.B. and Rodrigues,
C.E.C.,2017. Cocoa shell and its compounds
Applications in the Food Industry.Trends in
Food Science & Technology. S0924-2244.
Peleteiro, S., Santos, V., Garotte, G. And Parajo,
J.C., 2016. Furfural Production from Eucalyptus
Wood using an Acidic Ionic Liquid.Carbohydrate
Polymers. 146: 20-25.
Stein, T.V., Grande, P. M., Leitner, W. and Maria, P.
D. D., 2011. Iron Catalyzed Furfural Production
in Biobased Biphasic Systems: From Pure
Sugars to Direct Use of Crude Xylose Effluentsas
Feedstock.ChemSusChem. 4: 1592-1594
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
164
