
The AAS Method for Arsen Analysis in Cabbage in the Area of
Sinabung Post Eruption
Boby Cahyady
1*
, Suharman
1
, Muhammad Taufik
1
and Fitri H Hasibuan
2
1
Chemistry Department, Faculty of Mathematic and Natural Science, Universita Sumatera Utara, Medan 20155,
Sumatera Utara, Indonesia
2
Postgraduate Chemistry Study Program, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara,
Jl. Bioteknologi No. 1 Kampus USU, Medan, Indonesia
Keywords: Sinabung, AAS, Arsenic, Lava, Eruption.
Abstract: Sinabung erupts will emit hot clouds. Flowing lava will bring considerable heat. Negative Impact after the
eruption of this mount is the exposure of cauliflower plants around the eruption site by Arsenic. This study
aims to determine of Arsenic from various types of cabbage (white, purple, green) exposed to the Sinabung
area after eruption using the Atomic Absorption Spectrophotometry (AAS) method. The sample used is
stem from white, purple, and green cabbage. Preparation of green cabbage samples in analytical laboratory
Universitas Sumatera Utara. Sampling uses simple random sampling technique. Process of destruction have
been developed and analysis using the Atomic Absorption Spectrophotometry (AAS) method used standard
solution at 0.05, 0.10. 0.150. 0.20. and 0.250 μg / mL. The wavelength used is 193.7 nm. The results of the
analysis showed that the amount of Arsenic contained in white, purple and green cabbage stems was 0.0072,
0.0043, and 0.0082 μg / mL respectively.
1 INTRODUCTION
Mount Sinabung is a Pleistocene to Holocene
stratovolcano. It is located in a relatively cool area
on a fertile plateau with mountains bounding the
north. The summit crater of the volcano has a
complex, longer form due to vents migrating on the
N-S line. The 2460 meter high andesitic to dacitic
volcano comes from the Sunda Arc, which is created
by the subduction of the Indo-Australian Plate under
the Eurasian Plate. The Andaman Islands are on the
North-Northwest bound of the arc while the Banda
Arc is on the East. Sinabung has a total of four
volcanic craters, one of them being active currently
(Endang Tri Wahyuni, Sugeng Triyono, 2012).
The various activities of Mount Sinabung cer-
tainly have positive and negative impacts on the
population around Mount Sinabung. There are nega-
tive impacts that can be directly felt by residents
around Mount Sinabung, for example when Mount
Sinabung erupts it emits hot clouds and lava which
flow with enough heat / energy. Gray-white volcanic
dust has covered the forest, villages and surrounding
agricultural land, so it is necessary to examine the
danger of volcanic dust to the health of local resi-
dents of agricultural crops and livestock of local
residents, agricultural crops and livestock. Volcanic
dust after the eruption of Mount Sinabung produces
Arsenic and various heavy metals that have an im-
pact on the quality of agricultural products including
cabbage (Nain Felix Sinuhaji, 2011).
Arsenic is the most toxic chemical and metalloid
found in nature and is an important element of con-
cern because it can cause toxicity and carcinogens,
even at low concentrations (López et al., 2012).
Exposure to arsenic in humans can be in inorganic
and organic forms. The presence of arsenic in the
environment can occur as natural substances and
contamination from human activities. Arsenic can be
found in water, air, food, and soil including from
volcanic eruptions, contamination from mines, use
of pesticides and fertilizers. The toxicity of arsenic
has been widely known, but depends on the form of
organic or inorganic arsenic compounds (Hazimah
dan Nurlinda Ayu Triwuri, 2018).
Inorganic arsenic is soluble in water or in the
form of gases and exposed to humans, other than
that it is the most toxic element and is found in soil,
air and water. Natural arsenic is produced from
volcanic eruptions that can release around 3000 tons
Cahyady, B., Suharman, ., Taufik, M. and Hasibuan, F.
The AAS Method for Arsen Analysis in Cabbage in the Area of Sinabung Post Eruption.
DOI: 10.5220/0008920102490252
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 249-252
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
249

every year and can react with halogens, concentrated
peroxide and hot alkalis. The compounds of arsenic
with oxygen, chlorine and sulfur are called inorganic
arsenic, while the compounds of arsenic with carbon
and hydrogen are called organic arsenic. Arsenic
compounds are used in insecticides and as doping
materials in semiconductors. Arsenic is also used to
harden some lead metals (Joshi et al., 2016). Base
on research conducted by the USA Organic Trade
Association (2015) stating that rice, food and
organic food does not guarantee that organic
products do not have and are not exposed to arsenic.
However, many people do not know this, even
believing that organic food and food have nutrients
and advantages for health and are free of arsenic
compared to inorganic food. This assumption is very
wrong, because organic food still contains arsenic
levels as in organic rice. A study conducted by Xue,
et al. (2010) to determine the amount of arsenic
daily intake in the community in America, obtained
total levels of arsenic exposure through food by 0.38
μg / kg body weight / day. These results were 14
times higher than the amount of arsenic exposure
from drinking water. From the results obtained it can
be concluded that Americans are more exposed to
arsenic through food than through drinking water
(Joshi et al., 2016). Exposure to arsenic in humans
has caused many diseases in humans that have
occurred and have been evaluated by countries
throughout the world. These adverse effects on
health have led countries such as Canada to increase
safety of exposure to arsenic in drinking water by
reducing arsenic requirements in drinking water
from 50 µg / L to 25 μg / L (Mukherjee et al., 2006).
Arsenic compounds in inorganic forms are more
toxic than organic. Arsenic is carcinogenic because
long-term exposure can result in an increased risk for
various carcinomas including the skin, bladder, lungs,
kidneys, liver and prostate. The effects of arsenic are
related to changes in gastrointestinal, cardiovascular,
hematological, pulmonary, neurological, immunolog-
ical, reproductive and long-term effects of arsenic can
cause cancer (Joshi et al., 2016). According to the
International Agency for Research on Cancer (IARC),
arsenic is included in the first class as a carcinogen
and states that arsenic can cause lung, skin and blad-
der cancer in humans without a minimum threshold
where small amounts of arsenic can be harmful to
human health (López et al., 2012).
Evi Ekayanti Ginting (2018) has examined the
analysis of metal arsenic (As) in rice. The results
showed that the highest levels of arsenic metal in rice
circulating in the city of Medan were 3.71 mg / kg in
brown rice, 3.40 mg / kg in brown rice, 0.33 mg / kg
in white rice and 0.13 mg of black rice / kg. (Ulfa,
2015). Ridwan, M. H. (2012) analyzed Arsenic (As)
metals in spinach showing the results that in Green
spinach (Amaranthus tricolor) a concentration of 0. 35
mg / kg was obtained While the red spinach (Blitum
rubrum) obtained a concentration of 0.40 mg / kg.
Arsenic (As) compounds are thought to be exposed to
cabbage farmers who are exposed to the eruption of
Mount Sinabung. In this study, purple, green and
white cabbage leaves were analyzed using the Atomic
Absorption Spectrophotometry (AAS) method.
2 MATERIALS AND METHODS
2.1 Collecting and Preparation Sample
In this work, the population was green cabbage veg-
etables in the area after the eruption of Mount
Sinabung. Sampling uses simple random sampling
technique (random sampling). In this method the
sample members choose directly from the entire
population without being calculated based on the
population because they consider having the same
number to be chosen. So this way is considered a
large group, while samples are taken to represent the
population. 100 g of cabbage is washed with running
water and drained dry. Then blend until smooth.
2.2 Destruction Process
The white, purple, and green cabbage @0.5 g) put
into the vessel, added 5 mL of 65% HNO
3
and 3 mL
of 37 % HCl. Then let stand for 10 minutes so that
the sample dissolves. Vessel is inserted into the
microwave at 180
0
C for 30 minutes until destruction
occurs perfectly which is marked by obtaining clear
liquid. Then the destruction results are cooled and
put into a 50 mL volumetric flask and filled with
demineralized acua to 50 mL and filtered used
Whatmann paper No. 41.
2.3 Calibration Curves Used AAS
Instrument
The standard Arsenic solution (1000 μg / mL) was
piped 10 mL and then put into a flask measuring 100
mL and filled to the mark line with aquadest (con-
centration 100μg / mL). Then piped 5 mL and then
put it in a flask measuring 500 mL and filling it up
to the mark line with aquadest (Concentration of 1
μg / mL). Retweeted (0. 0.5; 1; 1.5; 2; 2.5) mL was
put into a flask measuring 100 mL and filled to the
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
250
mark line with aquadest until concentration (0;
0.005; 0.01; 0.015 ; 0.02; 0.025) μg / mL) and meas-
ured the absorbance by atomic absorption spectropo-
tometer at a wavelength of 193.7 nm. The absorb-
ance value and concentration will be plotted to ob-
tain the calibration curve and then calculated the
regression equation.
2.4 Determination of Arsenic Levels
The solution of the destruction of the sample, HCl,
NaBH4, is flowed by the pump to manifolid to mix
and forward to the coli (circle) to form a hydride.
Steering is measured using an atomic absorption
spectrophotometer at a 193.7 nm wavelength
equipped with Vapor Hydride Generation Acessories.
3 RESULTS AND DISCUSSIONS
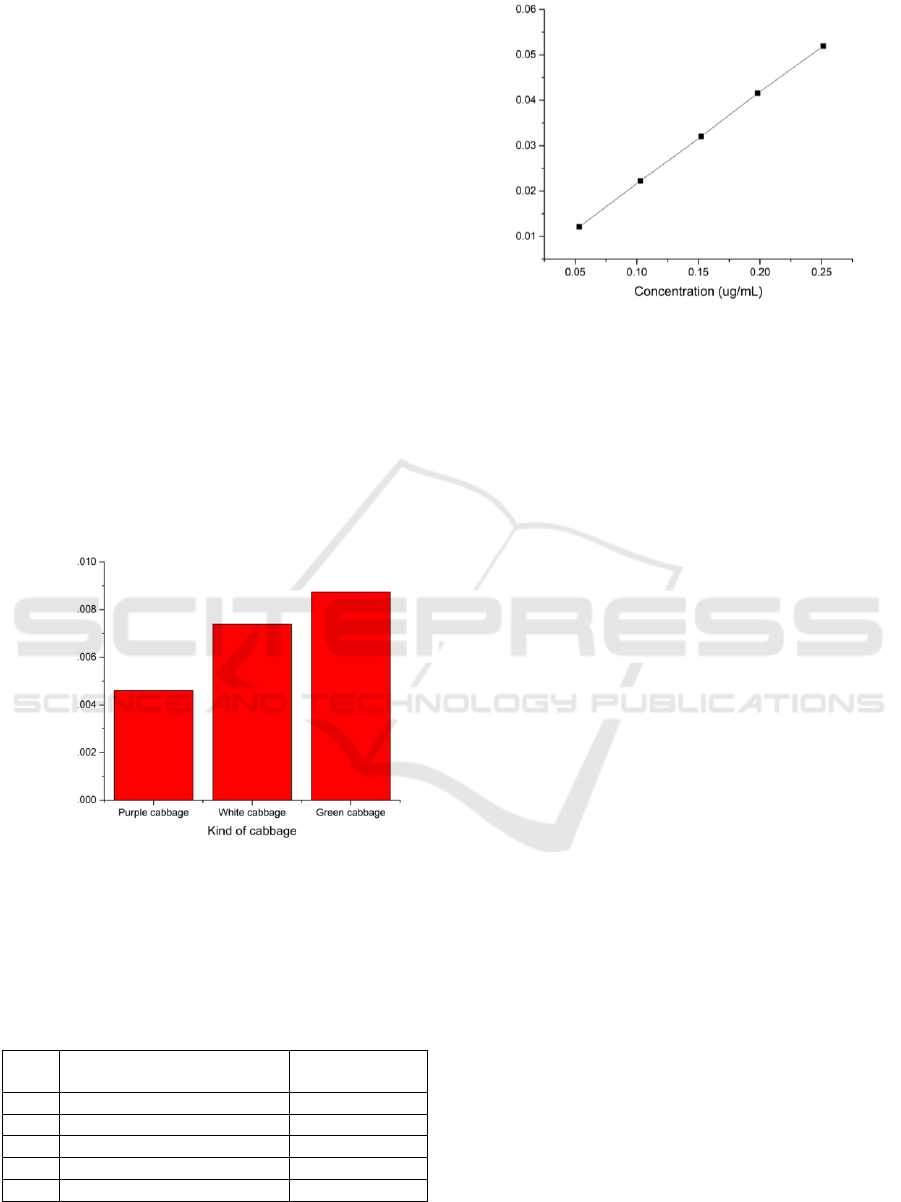
The results of the study of Arsenic levels exposed to
white, purple, and green cabbage are as follows
Figure 1.
Figure 1: Arsenic concentration in cabbage.
Standard Solution Absorption Measurement
Results on Metal Arsenic (As) nn Wavelength of
193.7 nm are follows Table 1 and the last squre
equation at Figure 2.
Table 1: Standard solution Absorption at 193,7 nm.
No.
Concentration (μg / mL )
Absorbantion
(A)
1
0.05
0.0121
2
0.10
0.0221
3
0.15
0.0320
4
0.20
0.0412
5
0.25
0.0522
Figure 2: Concentration vs Absorbantion.
This research was conducted with analyze the
metal content of arsenic (As) in white, purple, and
green cabbage found in Mount Sinabung post
eruption using atomic absorption spectrophotometry
(AAS). Advantages of the method Atomic
Absorption Spectrophotometer (SSA) compared
with ordinary spectrophotometer that is specific, low
detection limit of solution the same can measure
different elements, measurements directly towards
the sample, output can be read directly, enough
economical, can be applied to many types of
elements, limit levels Determination of area from μg
/ mL to %. The purpose of destruction is done is to
overhaul the organic compounds contained within
sample, so that it will be obtained last simpler
compound the remaining HNO
3
is removed by
means of heated on a hot plate inside fume hood to
prevent inhalation of NO
2
(poisons). Then sample
that has been destroyed diluted with distilled water
until 50 mL, then filtered using Whatman filter
paper no. 41. Until a clear solution is obtained.
Filtrate obtained is used for analysis of arsenic metal
content using a spectrophotometric device atomic
absorption at length wave 193.7 nm (As).
Cabbage are a great source of vegetable protein
and many contains vitamins A, B and C, especially
in the seeds. Several types of cabbage which is
cultivated among green cabbage, compost
beanspurple cabbage, and white cabbage. Cabbage
have important potential in the framework
fulfillment of nutrition, foreign exchange earnings,
improvement of community welfare, and
improvement in farmer's income. The results of the
eruption of Mount Sinabung emitted smoke thick
black with sand, and volcanic dust covering
thousands of hectares of farmers' crops which are
under a six kilometer radius covered in sand dust.
Volcanic dust causes many farmers' plants on the
slopes of the mountain to die and be damaged. It is
The AAS Method for Arsen Analysis in Cabbage in the Area of Sinabung Post Eruption
251

estimated that an area of 15341 hectares of displaced
agricultural crops from Mount Sinabung is
threatened with crop failure and one of which is the
exposure of arsenic specific metals.
4 CONCLUSIONS
Arsenic from various types of cabbage (white,
purple, green) exposed to the Sinabung area after
eruption using the Atomic Absorption
Spectrophotometry (AAS) method was
determinated. Atomic Absorption
Spectrophotometry (AAS) method was applicated
used standard solution at 0.05, 0.10. 0.150. 0.20. and
0.250 μg / mL. The wavelength used is 193.7 nm.
The results of the analysis showed that the amount
of Arsenic contained in white, purple and green
cabbage stems was 0.0072, 0.0043, and 0.0082 μg /
mL respectively.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Rector of Uni-
versity of Sumatera Utara for the financial support
via Penelitian Dasar Talenta Project 2019
REFERENCES
Endang Tri Wahyuni, Sugeng Triyono, dan S., 2012.
Determination of Chemical Composition of Vulcanic
Ash from Merapi Mountain Eruption. J. Mns. dan
Lingkung. 19, 150–159.
Evi Ekayanti Ginting, 2018. Analisis Arsen pada Berbagai
Jenis Beras yang Beredar di Kota Medan dengan
Spektrofotometri Serapan Atom.
Hazimah dan Nurlinda Ayu Triwuri, 2018. Analisis
Kandungan Arsenik (As) dan Cianida (Cn) Depot Air
Minum Isi Ulang Di Kota Batam. J. Rekayasa Sist.
Ind. 3, 129–133.
Hilda, L., Si, M., 2014. Analisis Kandungan Lemak Babi
dalam produk pangan di Padangsidimpuan secara
kualitatif dengan menggunakan Gas Kromatografi
(GC). Tazkir 9, 1–15.
Joshi, T., Gaur, G., Agarwal, M., Singh, T., Gauba, P.,
2016. Arsenic Toxicity. J. Chem. Pharm. Researc 8,
240–245.
López, D. L., Bundschuh, J., Birkle, P., Aurora, M.,
Cumbal, L., 2012. Arsenic in volcanic geothermal
fluids of Latin America Dina. Sci. Total Environ. 429,
57–75. https://doi.org/10.1016/j.scitotenv.2011.08.043
Mukherjee, A., Das, B., Rahman, M. M., Chakraborti, D.,
2006. Arsenic Contamination in Groundwater : A
Global Perspective with Emphasis on the Asian
Scenario. J Helth Popul Nutr 1, 142–163.
Nain Felix Sinuhaji, 2011. Analisis Logam Berat Dan
Unsur Hara Debu Kabupaten Karo Vulkanik Gunung
Sinabung.
Taufik, M., Taufik, M., Wanto, R., Cibro, S. R., Ardilla,
D., Razali, M., Tarigan, D. M., 2017. Studi
Pendahuluan Maserasi Coupling Elektrosintesis dalam
Mengekstraksi Nikotin Yang Terkandung Dalam
Puntung Rokok, in: Seminar Nasional Kimia Unmul
2017.
Ulfa, A. M., 2015. Identifikasi Boraks Pada Pempek Dan
Bakso Ikan Secara Reaksi Nyala Dan Reaksi Warna
Ade. J. Kesehat. HOLISTIK 9, 151–157.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
252
