
Quality Improvement of Liquid Smoke of Coconut Shell
by Tar Scrubber
Reka M. Sari
1
, Saharman Gea
2*
, Basuki Wirjosentono
2
and Sunit Hendrana
3
1
Postgraduate Chemistry Study Program, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara,
Jl. Bioteknologi No. 1 Kampus USU, Medan, Indonesia
2
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Jl. Bioteknologi
No. 1, Medan 20155, Indonesia
3
Research Centre for Chemistry, Indonesian Institute of Sciences, Kawasan Puspiptek, Gedung 452, Tangerang Selatan,
Banten 15314, Indonesia
malikabdul4329@gmail.com, rachmadfauzi8888@gmail.com
Keywords: Coconut Shell, Liquid Smoke, Quality of Liquid Smoke, Tar Scrubber.
Abstract: One of the most challenging issues concerning to the pyrolysis of liquid smoke coconut shell is the presence
of tar. The acidity, water content, density and content analysis of liquid smoke using coconut shell which
produced at 500
o
C using pyrolysis reactor equipped with a tar scrubber were compared to liquid smoke
produced conventionally. Characterization of acidity of the liquid was measured by pH meter by adhering to
ASTM E70, water content by using volumetric Karl Fischer titration method following ASTM E203 and
ASTM D1744 method, the density by AOC 1995, and dichloromethane extracts content by gas
chromatography-mass spectrometry (GC-MS). The results showed that the pH range of liquid smoke was
pH 2.4 to 2.6 with the water content of 89.970 to 94.593%, and the density of 1.0118 to 1.0036 g/mL. The
carbonyl and phenol content were 5.05 to 6.473% and 85.057 to 90.024% respectively. From the results can
be concluded that the quality of liquid smoke produced by tar scrubber is better than the quality of liquid
produced by conventional method.
1 INTRODUCTION
Coconut shell liquid smoke is a result of pyrolysis of
coconut shell or condensation of steam distillation.
The constituents of the liquid smoke are obtained
from thermal degradation reactions of cellulose,
hemicellulose, and lignin. Hemicellulose owned the
highest CO
2
, lignin generated the highest CH
4
and
cellulose produced the highest CO characterized by
the largest HHV (Zhao, Jiang and Chen, 2017).
In the process of pyrolysis also produced liquid
smoke, tar and non-condensable gases. Liquid
smoke which is a by-product of the charcoal
industry has high economic value when compared to
being discharged into the atmosphere. Liquid smoke
is obtained from dew condensation resulting from
decomposition of organic compounds during the
pyrolysis process. The content of liquid smoke from
pyrolysis is phenol compound of 90.75%, carbonyl
3.71% and alcohol 1,81%, the compound is
antimicrobial which can preserve food (Hadanu and
Apituley, 2016). The application of liquid smoke is
mainly associated with the functional properties of
liquid smoke, including as an antioxidant,
antibacterial, antifungal, and its potential in forming
the product.
Antimicrobial properties can inhibit the activity
of spoilage and spoilage microbes in food so that it
can extend the shelf life of food products (Olatunde
and Benjakul, 2018). In addition, liquid smoke can
also have an effect on distinctive taste, color, and
aroma. Some types of agricultural waste such as
corn cobs, rice husks, peanut shells, coconut shells,
coconut fiber, mangrove wood, pine and others
contain phenols and antibacterial properties that can
preserve and give flavor to food products (Dungani
et al., 2016).
Liquid smoke produced from coconut shell needs
further processing because it contains higher levels
of benzopyridene containing toxic thus liquid smoke
from coconut shell is not yet suitable for use
(Budaraga et al., 2016). The major proportion of
262
M. Sar i, R., Gea, S., Wirjosentono, B. and Hendrana, S.
Quality Improvement of Liquid Smoke of Coconut Shell by Tar Scr ubber.
DOI: 10.5220/0008920802620265
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 262-265
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

commercial full-strength liquid smoke to be
composed of water (11–92%), tar (1–17%), acids
(2.8–9.5%), carbonyl containing compounds (2.6–
4.6%) and phenol derivatives (0.2–2.9%) (Baltes et
al., 1981). Further treatment is needed to reduce
levels of toxic compounds, eliminate tar, increase
liquid smoke yield and its quality.
The general method used in removing tar from
liquid smoke is redestilation. Redestilation is the
process of purifying liquid smoke based on
differences in the boiling point of liquid smoke.
There have been many studies doing liquid smoke
redestilation (Darmadji, 2002; Budaraga et al., 2016;
Ketut Budaraga et al., 2016). At present, the method
offered in removing tar is wet scrubber because it is
simple, economical and high efficiency. Some
studies use this method for removing tar in syngas.
The objective of this study was to determine
important chemical characteristics of a full-strength
liquid smoke from tar scrubber. The chemical
volatile and semi-volatile constituents of these
product were identified using gas chromatography-
mass spectrometry (GC-MS) analysis. pH, acidity,
density were also determined.
2 MATERIALS AND METHODS
2.1 Materials
Raw material used in this study was coconut shell
from coconut shell charcoal industry, Johor, Medan,
Indonesia.
2.2 Equipment
In this study, the equipment used were a set of
pyrolysis tar scrubber reactor, pH meter,
pycnometer, analytical balance, Karl Fischer
coulometric titrator and GC-MS.
2.3 Liquid Smoke Production
Production of liquid smoke was done by pyrolysis.
The pyrolysis reactor was equipped with a tar
scrubber which could be charged with as much as
500 kg of material. The reactor cover was connected
by a pipeline to the cooling tubes used to condense
the fumes and generate the liquid smoke. After all
materials were inserted into the furnace, it was
closed, the condenser was set, and the cooling tube
was streamed with cold water. Pyrolysis was carried
out at a temperature 500°C for 8 hours.
2.4 Characterization
Acidity of liquid smoke was characterized by pH
meter, water content by using volumetric Karl
Fischer titration, the density by AOC 1995, and
dichloromethane extracts content by gas
chromatography-mass spectrometry (GC-MS).
3 RESULTS AND DISCUSSION
3.1 Liquid Smoke Chemical
Components
Liquid smoke compositions are obtained from
pyrolysis of coconut shell. The traditional liquid
smoke manufacturing saw dust pyrolyzed in
temperature ranges of 350-600
o
C and under
atmospheric pressure conditions. In this research, the
liquid smoke was obtained from thermal
degradation reactions of cellulose, hemicellulose,
and lignin. From a proximate components
standpoint, the three major components of coconut
shell are cellulose, hemicellulose, and lignin . The
pyrolysis of lignin was reported at around 310-500
o
C and yielded the major source of phenols
(Martinez et al., 2007). In the other research, the
hemicellulose yielded furan, furan derivatives, and a
series of aliphatic carboxylic acids (Siskos et al.,
2007).
The quality of liquid smoke is very dependent on
the composition of chemical compounds contained
in liquid smoke. The compounds contained in liquid
smoke are strongly influenced by the conditions of
pyrolysis and types of raw materials (Budaraga et
al., 2016). This is due to the large levels of cellulose
and hemicellulose from each ingredient. Cellulose
pyrolysis takes place in two stages, namely the first
stage is an acid hydrolysis reaction followed by
dehydration to produce glucose, while the second
stage is the formation of acetic acid and homologous
together with water and a small amount of furan and
phenol (Collard and Blin, 2014).
Identification of phenol and carbonyl were detected
gas chromatography-mass spectrometer in
dichloromethane fractions. Results were shown on
Table 1 in which phenol ranged from 85.057 –
90.024% while carbonyl from 5.05 – 6.473%. This
shown that there were an increase in the chemical
components produced by pyrolysis tar scrubber.
Quality Improvement of Liquid Smoke of Coconut Shell by Tar Scrubber
263
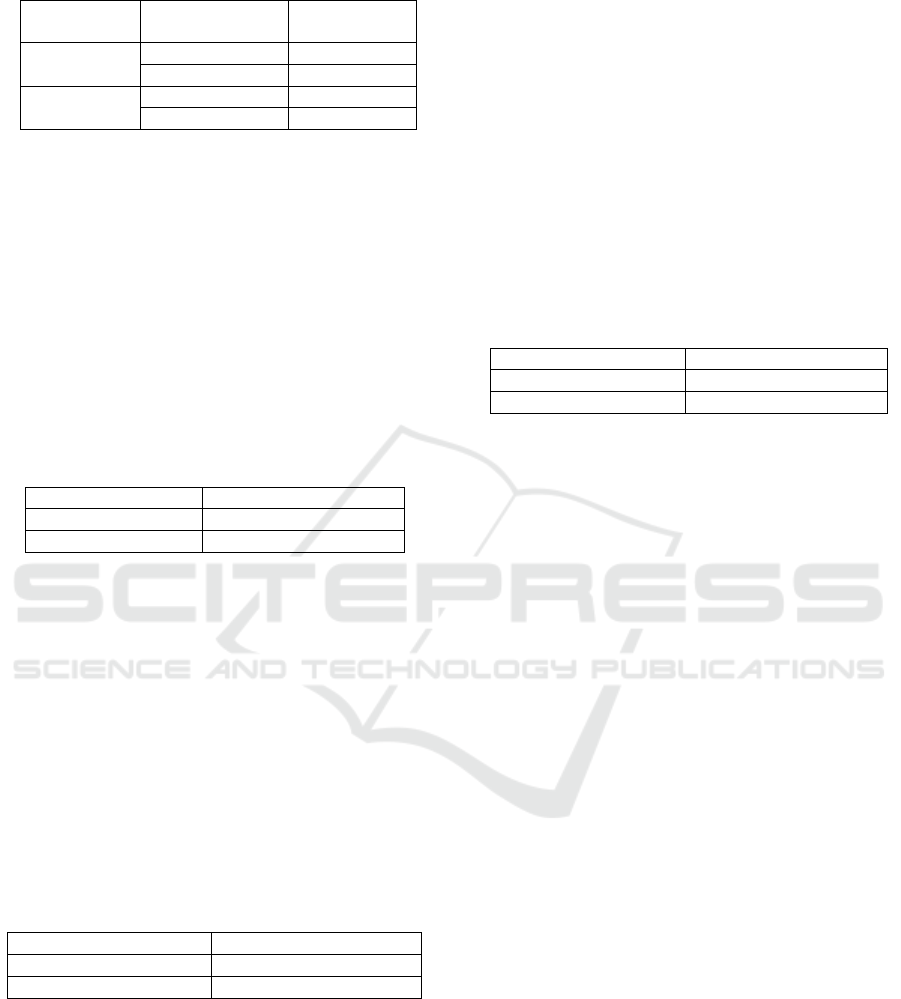
Table 1: Chemical Compenents of Liquid Smoke.
Method
Chemical
Components
(%) Area
Tar Scrubber
Phenol
90.024
Carbonyl
6.473
Conventional
Phenol
85.057
Carbonyl
5.05
3.2 Density
Specific gravity is the relative ratio between the
density of a substance and the density of pure water.
The Table 1 below shows the effect of the results of
pyrolysis by conventional methods and tar scrubbers
on the specific gravity of liquid smoke.
From the Table 2 it can be seen that the specific
gravity of liquid smoke from the tar scrubber at a
temperature of 500⁰C has a higher value than the
value of the specific gravity of liquid smoke in the
conventional method.
Table 2: Density of Liquid Smoke.
Method
Density (g/mL)
Tar Scrubber
1.0118
Conventional
1.0036
3.3 Acidity
Acetic acid compounds are classified as acid
compounds that affect the pH of liquid smoke and
taste and aging of smoke products. In addition,
phenol levels also affect the pH of liquid smoke
because phenol has acidic properties which are the
influence of aromatic rings. The pH value is one of
the quality parameters of liquid smoke produced.
The pH measurement was carried out on the liquid
smoke from the pyrolysis results and the distillation
results from each material with the results obtained
as shown in Table 3.
Table 3: Acidity of Liquid Smoke.
Method
Acidity (pH)
Tar scrubbe
2.4
Conventional
2.6
The tendency to decrease the pH of liquid smoke
is because the content of Acetic acid and phenol
compounds increases after distillation. The higher
the total phenol level in liquid smoke, the lower the
pH value or more acidic.
3.4 Water Content
From the Table 4. below it can be concluded that the
higher the pyrolysis temperature, the lower the water
content contained in liquid smoke. At the
conventional method was lower temperature than tar
scrubber method. The water content contained in
liquid smoke was quite large because this
temperature was evaporated and condensed.
Whereas at temperatures of 500⁰C there is a
decomposition of components of organic matter
contained in wood to produce liquid smoke with a
more concentrated color and water content which
tends to decrease.
Table 4: Water Content of Liquid Smoke.
Method
Water Content (%)
Tar Scrubber
89.970
Conventional
94.593
4 CONCLUSIONS
Optimum condition obtained through hydrogel
Generally, the manufacture of liquid smoke by the
pyrolysis tar scrubber method can improve liquid
smoke quality. In addition, the most phenol and
carbonyl contents were produced in tar scrubber
method.
ACKNOWLEDGEMENTS
The authors would like to thanks to the Ministry of
Research, Technology and Higher Education for
funding support by scheme of PMDSU 2017.
REFERENCES
Baltes, W., Reiner, W., Ingeborg, S., Helmut, B., Lazlo,
T., 1981. Ingredients of Smoke and Smoke Flavor
Preparations. in The Quality of Foods and Beverages.
1-19.
Budaraga, K., Arnim., Yetti, M., Usman, B., 2016. Liquid
smoke production quality from raw materials variation
and different pyrolysis temperature. International
Journal on Advanced Science, Engineering and
Information Technology. 6.
Collard, F. X. and Blin, J., 2014. A review on pyrolysis of
biomass constituents: Mechanisms and composition of
the products obtained from the conversion of cellulose,
hemicelluloses and lignin. Renewable and Sustainable
Energy Reviews. 38, 594-608.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
264

Darmadji, P., 2002. Optimation of Liquid Smoke
Purification by Redistilation Method. Jurnal Teknol.
dan Industri Pangan. 13.
Dungani, R., Myrtha, K., Subyakto., Sulaeman., Dede, H.,
Hadiyane., 2016. Agricultural waste fibers towards
sustainability and advanced utilization: A review.
Asian Journal of Plant Sciences. 15(1-2), 42-55.
Hadanu, R. and Apituley, D. A. N., 2016. Volatile
Compounds Detected in Coconut Shell Liquid Smoke
through Pyrolysis at a Fractioning Temperature of
350-420°C. Makara Journal of Science. 20(3), 95-100.
Ketut Budaraga, I., 2016. Analysis of liquid smoke
chemical components with GC MS from different raw
materials variation production and pyrolysis
temperaturelevel. International Journal of ChemTech
Research.
Martinez, O., Salmeron., Jesus, G., Maria, C., 2007.
Textural and physicochemical changes in salmon
(Salmo salar) treated with commercial liquid smoke
flavourings. Food Chemistry. 100(2), 498-503.
Olatunde, O. O. and Benjakul, S., 2018. Natural
Preservatives for Extending the Shelf-Life of Seafood:
A Revisit. Comprehensive Reviews in Food Science
and Food Safety.17(6), 1595-1612.
Siskos, I., Ilias, Zotos., Anastasios, M., Styliani, T.,
Roussa., 2007. The effect of liquid smoking of fillets
of trout (Salmo gairdnerii) on sensory, microbiological
and chemical changes during chilled storage. Food
Chemistry. 101(2), 458-464.
Zhao, C., Jiang, E. and Chen, A., 2017. Volatile
production from pyrolysis of cellulose, hemicellulose
and lignin. Journal of the Energy Institute. 90.
Quality Improvement of Liquid Smoke of Coconut Shell by Tar Scrubber
265
