
Magnesium Impregnated Silica Mesoporous Prepared using Ester
Ricinoleic as Template for the Esterification
Andriayani
1,2*
, Marpongahtun
1
, Yugia Muis
1
1
Department of Chemistry, Faculty of Mathematics and Natural Science, Universitas Sumatera Utara, Medan, Indonesia,
20155
2
Pusat Kajian IPTEKS Minyak Atsiri Eucaplytus Universitas Sumatera Utara, Medan, 20155, Indonesia
Keywords: mesoporous silica, ester ricinoleic, template, magnesium.
Abstract: Synthesis of mesoporous silica material was carried out using ricinoleate ester as a template. Mesoporous
silica products were characterized using FT-IR, XRD, SEM and nitrogen adsorption. Mesoporous silica
material was impregnated with magnesium nitrate, impregnation products were characterized using FT-IR,
XRD, SEM and BET analysis. MgO impregnated silica mesoporous is applied as a catalyst in the reaction
of esterification of castor oil to ricinoleate ester.
1 INTRODUCTION
Porosity greatly influences the physical properties of
a material such as density, heat conductivity,
strength and others (Schubert, Ulrich S. and Husing,
2005). The synthesis technique of mesopore material
(2-50 nm pore diameter) is currently developing
rapidly because mesopore material has unique
properties, such as a more regular pore structure,
large surface area and uniform pore size distribution.
So much applied as catalysts (Li et al., 2011),
adsorbents (Yan et al., 2006), drug delivery
(Slowing et al., 2008), biosensors (Hasanzadeh et
al., 2012), optics (Kumari and Sahare, 2013) and
others.
The synthesis technique of mesoporous material
is carried out by combining inorganic components as
material and organic components such as surfactants
functioning as pore printers (templates). The pore
will be obtained after the organic component has
been removed by calcination.
In this paper, tetraethylortosilicate (TEOS) is
used as a source of silica, risinolet methyl ester as a
template is made by extracting Ricinus communis
seeds that grow in wild forests in the North
Sumatera Karo region. Also used are 3-
aminopropyltrimethoxysilane (APMS) as a co-
structure directing agent (CSDA). The alkoxylane
group from CSDA will condense with inorganic
precursors and the ammonium group will interact
electrostatically with anionic surfactants. The
interaction that occurs between surfactant and
silicate is S-N
+
I
-
where N
+
is CSDA.
In the previous study (Andriayani et al., 2013)
have been done synthesized of material silica using
sodium risinoleate as a template by varying the
addition of HCl 0,1N. In this paper we impregnate
MgO on silica mesoporous which is made using
methyl ester ricinoleate as a template and analyzed
using FT-IR, XRD, SEM and BET. The mesoporous
silica impregnation product was applied as a catalyst
in the reaction of castor oil eseterification to
ricinoleate ester. Given the increasingly limited
fossil fuels, ricinoleic esters can be an alternative to
fuels sourced from plants.
2 MATERIALS AND METHODS
2.1 Materials
Tetraethylorthosilicate (TEOS, 98%) and 3-
aminopropyltrimethoxysilane (APMS) were
purchased from Sigma Aldrich, methanol, and
hexane purcase from Emerc, methyl esther ricinoleic
acid (C
19
H
36
O
3
) obtained from Ricinus Communis
seed and deionized water obtained from PT Sumber
Aneka Karya Abadi. Jatropha seed oil is obtained
from Bratachem, Mg(NO
3
)
2
(Merck), n-hexene.
274
Andriayani, ., Marpongahtun, . and Muis, Y.
Magnesium Impregnated Silica Mesoporous Prepared using Ester Ricinoleic as Template for the Esterification.
DOI: 10.5220/0008921702740279
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 274-279
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

2.2 Characterizations
The obtained products were then subjected to
characterization by using X-ray diffraction (Philip
PW 1710), Fourier transform infrared (Shimadzu IR-
Prestige-21), scanning electron microscope (JEOL
JSM-7000F), transmission electron microscope
(JEOL JEM-1400) and adsorption desorption
isotherm (Quantachrome Auto¬sorb), Atomic
absorption spectroscopy (Shimadzu AA7000).
2.3 Synthesis of Silica Mesoporous
Material using Risinoleic Methyl
Ester as a Template
Methyl esters of ricinoleate (C
19
H
36
O
3
) of 4.52 g
(0.015 mol), 100 ml deionized water and 1.2 grams
of methanol were put into two neck flasks and
sterilized at room temperature for 30 minutes (mix
A). Then a mixture of 1.2 g (0.007 mol) APMS
(C
6
H
17
SiO
3
N) and 6.04 g (0.029 mol) TEOS (C
8
H
20
SiO
4
) was stirred for 10 minutes (mixture B).
The mixture (B) was added to the mixture (A)
and then stirred for 2 hours. Then let it sit in the
oven at 80
o
C for 3 days (36 hours) until a porous
solid is formed. The mixture is centrifuged and the
solids are separated and washed with deionized
water. The solid is dried at 50
C and then calcined
at 550
C for 6 hours. Silica mesoporous products
were then characterized using FT-IR, XRD, SEM
analysis and N
2
isotherm adsorption / desorption.
2.4 Impregnation of Mesoporous Silica
Material with Magnesium
Silica mesoporous material (0.75 gram) mixed with
Mg(NO
3
)
2
.6H
2
O (g) and added 25 mL of dry
methanol, then stirred at room temperature for 2
hours. The mixture is vacuumed to dry solids and
then solids are calcined for 12 hours at 550
o
C.
Mesoporous silica impregnation products were
characterization using FT-IR, XRD, AAS, BET and
SEM.
2.5 Application of Mesoporous Silica
Impregnation Products as
Esterification Catalyst
Mesoporous silica impregnation products (0.2 g),
methanol (p.a) (6.14 g) and castor oil (15 grams)
were put into a two neck flask. The mixture is stirred
with a magnetic stirrer for 4 hours at 80° C by reflux
method. The solid is separated from the reaction
mixture by filtering. The filtrate is extracted using n-
hexane and distilled water. Then the n-hexane phase
was vacuum and a pale yellow methyl ester product
of 10.59 grams or 70.6% yield was obtained. The
ricinoleate methyl ester product was characterized
using FT-IR and GC-MS.
3 RESULTS AND DISCUSSION
The silica mesoporous used to be applied as a
catalyst was obtained from one of the silica
mesoporous under the conditions of the preparation
of methanol addition variations without the addition
of 0.1M HCl. The reaction conditions for
mesoporous silica preparation using
tetraethylortosilicate (TEOS) as a source of silica,
methyl ester risinoleate obtained from esterification
of castor oil from castor beans (Ricinus communis)
as a template, using 3-aminopropiltrimethoxysilane
(APMS) as a co-structure directing agent and adding
methanol 1 2 grams without the addition of 0.1M
HCl. After maturing for 72 hours, the solid is
separated, washed, dried and to remove the template
calcined at 550
o
C for 6 hours a white solid is
obtained. Furthermore, it is characterized by FT-IR,
XRD, SEM and porosity analysis using BET.
Mesoporous silica is impregnated using
Mg(NO
3
)
2
in a dry methanol solvent, the solid is
separated, vacuum and calcined at 550
o
C for 12
hours. White solids were obtained as much as
0.6415 grams. Mesoporous silica impregnation with
Mg (NO
3
)
2
produced silica-MgO mesoporous (MS-
MgO). Magnesium oxide is attached to the surface
of the mesoporous silica material. AAS analysis
results showed that the Mg content contained in
mesoporous silica material was 1.3549 ppm.
Subsequently the solids were characterized using
FT-IR, XRD, SEM and porosity analysis using BET.
Functional group analysis using the FT-IR
spectrum of mesoporous silica that has not been
impregnated with silica mesoporous that has been
impregnated with MgO (Figure 1) shows the change
in functional groups in both materials. The
mesoporous silica spectrum before impregnation
(Figure 7 in black) showed an absorption peak at
3428.58 cm
-1
which was widening due to OH (strain
Si-OH) strain and supported the absorption peak at
964.41 cm
-1
due to streching (–SiO-H). The
absorption peak at 1103.28 cm
-1
is strong due to the
asymmetric streching of Si-O-Si and the wave
number at 810.10 cm
-1
is caused by the presence of
symmetrical Si-O-Si. The spectrum data is adjusted
to the literature: (Khalil, 2007; AlOthman and
Apblett, 2010; Liu et al., 2010; Zhao et al., 2011).
While the mesoporous silica spectrum that has been
impregnated by MgO shows the absorption peak at
3448.72 cm
-1
which was widened due to OH group
Magnesium Impregnated Silica Mesoporous Prepared using Ester Ricinoleic as Template for the Esterification
275
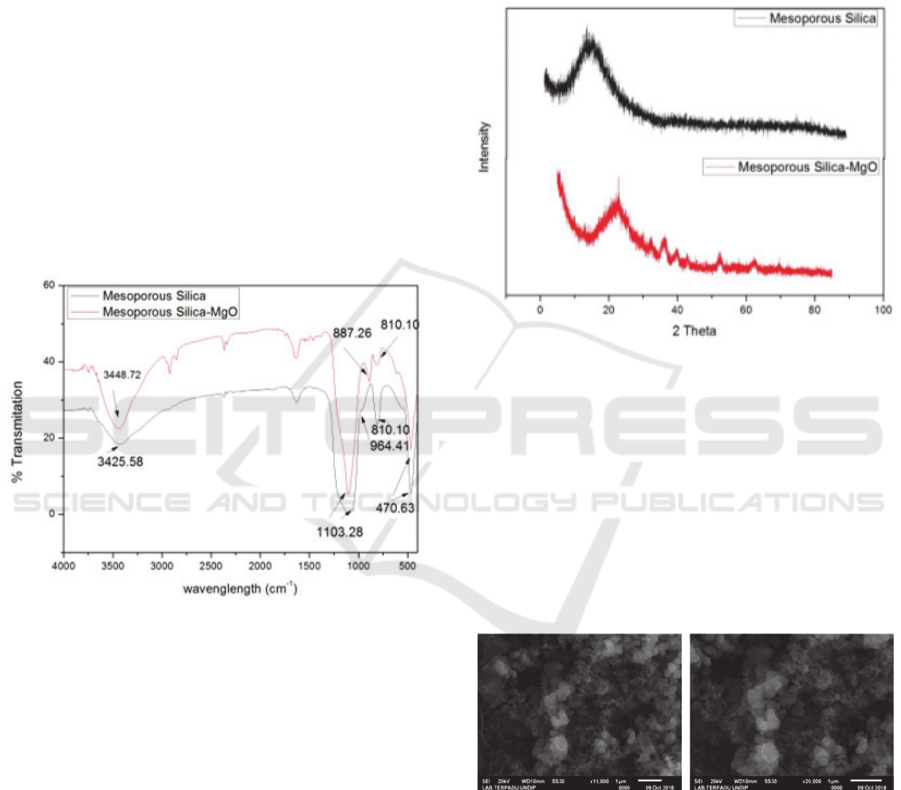
strain (Si-OH) and supported the absorption peak at
887.26 cm
-1
due to streching (–SiO-H). The
absorption peak at 1103.28 cm
-1
is strong due to the
asymmetric streching of Si-O-Si and the wave
number at 810.10 cm
-1
is caused by the presence of
symmetrical Si-O-Si groups.
The peak absorption of Si-OH groups is different
compared to mesoporous silica before being
impregnated where the peak shape is not too wide
and there is a shift in the wave number. This is due
to the surface of the silanol group which has been
impregnated with MgO. Likewise with the peak
caused by the Si-O-Si group there is a difference
compared to the mesoporous before digrafting such
as a sharp peak shape but not widening (slimmer)
with an intensity of 5.19 while for the mesoporous
silica the sharp peak shape widens and the intensity
is low 0.04. This is due to the mesoporous that has
been impregnated by the formation of Si-O-M bonds
(M = metal Mg) which is in the wave number 1000-
900 with a strong band (Smith, 1960).
Figure 1: Mesoporous Silica FT-IR Spectrum Before
Impregnation and After MgO Impregnation
Analysis of the mesoporous silica structure
before impregnation and after impregnation (Figure
2) shows the differences in the diffractogram of the
two materials. The mesoporous XRD diffractogram
of silica before impregnation (Figure 2 in black) has
only one diffractogram peak at an angle of 2 at
20.9865 the broad peak (broad) with a peak height
of 14.20. This shows that the material is
nanoparticles and amorphous structures that have
pores. This is consistent with data reported by
previous researchers (Park et al., 2006; Khalil, 2007;
Shah, Li and Ali Abdalla, 2009; Liu et al., 2010;
‘No Title’, 2011; Li et al., 2011; Zhao et al., 2011).
Whereas the mesoporous silica diffractogram that
has been impregnated with MgO (Figure 2 in red)
has several diffractogram peaks. Diffractogram at an
angle of 2 at 22.8454 with a peak that widened to
a height of 31.63 indicates that the material is
nanoparticles with a porous amorphous structure.
Whereas the diffractogram at 2 at 32, 35, 36,
39, 42 and 45 is the peak of the Mg metal
diffractogram which is impregnated on the silica
mesoporous surface. This proves the process of
mesoporous silica impregnation has taken place.
Figure 2: XRD Diffractogram of Mesoporous Silica
Mesopori Before Impregnation and After MgO
Impregnation
Mesoporous morphological analysis of the silica
before impregnation (Figure 3) using a scanning
electron microscope (SEM) magnification 15000
times and 20000 times showed that the material has
a mixed particle form, dominated by dispersed
spherical particles and some that form smaller
aggregates. Other particles in the form of sheets with
a small amount.
Figure 3: SEM Image of Mesoporous Silica Before MgO
Impregnation (left 15000 times and right 20000
times magnification)
Analysis of porosity of silica material before
impregnation and after impregnation of MgO
(Figure 5) shows the differences in adsorption
desorption isotherm graphs. Based on its hysterical
form, silica material approaches Type IV for silica
mesoporous according to the specific IUPAC
classification for silica mesoporous material. Graph
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
276
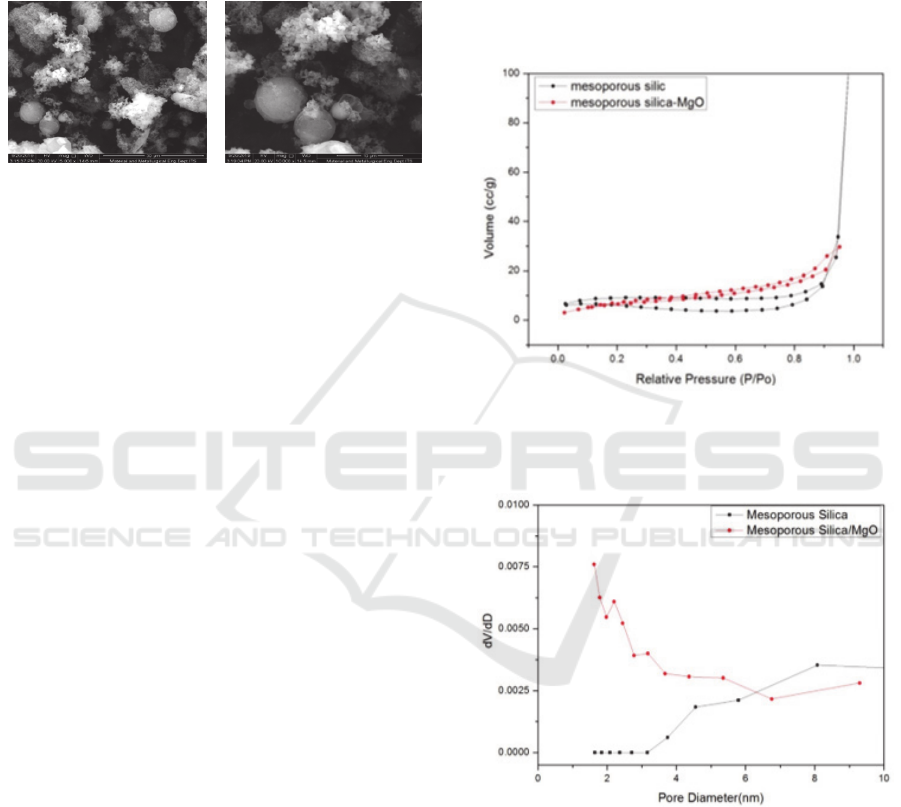
of silica meopore desorption adsorption before
impregnation (Figure 5 in black) lop hysteresis form
is Type H4, where the lop's hysteresis form is a bit
more complex because of the reversible micropore
filling area followed by multilayer physisorption and
capillary condensation. So lop H4 is the same as lop
H3 for non-micropore materials.
Figure 4: SEM Image of Mesoporous Silica After MgO
Impregnation (left 15000 times and right 20000
times magnification)
While the graph of adsorption desorption
isotherm of silica mesoporous material after MgO
impregnation (Figure 5 in red) the form of hysteresis
is Type H3 (Sing and Williams, 2004). This is due to
the shape of aggregate and plate particles,
characteristic desorption grooves and lower
approaching end points (closure points). Lop H3
does not have a plateau at high P/P0 values
(mesoporous volume is not well formulated), so
interpretation of high P/P0 values is more difficult.
Branch adsorption graphs on type H3 show that gas
adsorption only occurs on surfaces or manolayers so
that this shows that the obtained silica material can
be grouped also on Type II isotherm charts for non-
porous solids (Gregg S. J., and Sing, 1982). This is
due to the silica impregnated surface of MgO in the
pores that are already covered by MgO so that the
shape of the hysteresis loop resembles non-pore
solids.
Graph of mesoporous silica pore size distribution
before impregnation and silica mesoporous after
pregreated MgO (Figure 6) were calculated using the
Barret-Joyner-Halenda (BJH) method. The pore size
distribution of the two materials shows a difference.
The mesopore pore size distribution before
impregnation (Figure 5 in black) shows the pore size
distribution in the range of 1.64 nm - 8.105 nm.
While the pore size distribution of silica mesoporous
material that has been impregnated by MgO shows
that the pore size distribution is in the range of 1.61
nm - 9.31 nm.
The pore size distribution graph of the two
materials has a difference in the dV / dD value,
which is because there is a re-calcination treatment
for silica mesoporous MgO impregnated causing the
number of pores formed in the range of pore size
distribution of 1 nm - 6 nm to increase. Whereas the
dV/dD value of mesoporous material before
impregnation with the same (smaller) pore size
distribution range. But the dV/dD value in the pore
size distribution from 6-10 nm for mesoporous
materials impregnated with MgO is getting smaller
because the pores are covered with MgO, whereas
the silica mesoporous material before the
impregnation of dV/dD values in the same pore size
distribution range is smaller big because it's not
covered in metal.
Figure 5: Adsorption Graph Desorption of Silica
Mesoporous Isotherm Before Impregnation and
After MgO Impregnation
Figure 6: Graphs of Silica Mesoporous Pore Size
Distribution Before Imprgenation and After
MgO Impregnation
The silica mesoporous material impregnated by
MgO was tested for its catalytic activity in the
esterification reaction of castor oil. The catalytic
system of esterification reaction takes place under
heterogeneous conditions where mesoporous silica-
MgO is insoluble (remains solid). Such reaction
conditions are advantageous because they are easily
separated between the product and the catalyst. The
Magnesium Impregnated Silica Mesoporous Prepared using Ester Ricinoleic as Template for the Esterification
277

reaction was carried out at 80
o
C for 4 hours. After
the reaction is stopped, the catalyst solids are
separated and the filtrate is extracted with n-hexane
and washed with distilled water, after vacuum the
product has obtained a pale yellow methyl ester
product of 10.59 grams or a yield of 70.6%. The
ricinoleate methyl ester product was characterized
using FT-IR and GC-MS.
The formation of ricinoleate methyl ester product
was characterized using FT-IR and GC-MS. FT-IR
spectrum of methyl ester ricinoleate castor oil
esterification product (castor oil) using silica-MgO
mesoporous catalyst (Figure 7) shows the
appearance of widening at 3417.86 cm
-1
due to OH
groups in the carbon chain of methyl ester ester
risinoleate. While the sharp peak at 2854.65 cm
-1
is
caused by the vibration frequency of the -CH-
hydrocarbon chain group of methyl ester ricinoleate.
Another sharp peak at 1743.65 cm
-1
was caused by
the carbonyl methyl ester ricinoleate group.
Figure 7: FT-IR Spectra of Risinoleate FT-IR Spectrum
Transeterification of castor oil using mesoporous
silica-MgO-catalyzed methanol produces a mixture
of fatty acid methyl esters. This is due to the
distance that there are other fatty acids such as
ricinoleic acid, palmitic acid, stearic acid, linoleic
acid, oleic acid and others. So if esterified other fatty
acids might also be esterified. To find out the
composition of methyl esters formed from castor oil,
GC-MS analysis was performed. Through GC-MS
method, it can be known the percentage of methyl
esters of ricinoleate formed through the application
of silica-MgO mesoporous catalyst. The results of
GC-MS chromatogram of castor oil esterification
products with a heterogeneous reaction system
(Figure 8) can be seen that the risinoleate methyl
ester formed has a remaining 84.48% purity of
15.52% is a methyl ester from other fatty acids.
Figure 8: Chromatogram GC-MS of Risinoleate Esther
4 CONCLUSIONS
The mesoporous silica impregnation which was
made using methyl ester ricinoleate as a template
was successfully carried out. This can be proven by
the differences in analysis of mesoporous silica
products before being impregnated and after being
impregnated, it can be proven from FT-IR, XRD and
BET analysis. Its application as a catalyst in castor
oil esterification reaction results in ricinoleate ester
of 10.96 g (73.06%).
ACKNOWLEDGEMENTS
This research was funded by the DRPM
Republic of Indonesia Ministry of Research and
Technology Republic of Indonesia Fiscal Year 2019.
REFERENCES
AlOthman, Z. A. and Apblett, A. W. (2010)
‘Synthesis and characterization of a hexagonal
mesoporous silica with enhanced thermal and
hydrothermal stabilities’, Applied Surface
Science, 256(11), pp. 3573–3580. doi:
10.1016/j.apsusc.2009.12.157.
Andriayani, A. et al. (2013) ‘Synthesis of
Mesoporous Silica from Tetraethylorthosilicate
by Using Sodium Ricinoleic as a Template and
3-Aminopropyltrimethoxysilane as Co-Structure
Directing Agent with Volume Variation of
Hydrochloric Acid 0.1 M’, Advanced Materials
Research, 789, pp. 124–131. doi:
10.4028/www.scientific.net/AMR.789.124.
Gregg S. J., and Sing, K. S. W. (1982) Adsorpsi,
Surface Area and Porosity. Second Edi. London:
Academic Press.
Hasanzadeh, M. et al. (2012) ‘Mesoporous silica-
based materials for use in biosensors’, TrAC -
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
278

Trends in Analytical Chemistry, 33, pp. 117–129.
doi: 10.1016/j.trac.2011.10.011.
Khalil, K. M. S. (2007) ‘Cerium modified MCM-41
nanocomposite materials via a nonhydrothermal
direct method at room temperature’, Journal of
Colloid and Interface Science, 315(2), pp. 562–
568. doi: 10.1016/j.jcis.2007.07.030.
Kumari, S. and Sahare, P. D. (2013) ‘Optical studies
of fluorescent mesoporous silica nanoparticles’,
Journal of Materials Science and Technology.
Elsevier Ltd, 29(8), pp. 742–746. doi:
10.1016/j.jmst.2013.05.013.
Li, B. et al. (2011) ‘Preparation of MCM-41
incorporated with transition metal substituted
polyoxometalate and its catalytic performance in
esterification’, Microporous and Mesoporous
Materials. Elsevier Inc., 156(1), pp. 73–79. doi:
10.1016/j.micromeso.2012.02.017.
Liu, H. et al. (2010) ‘Synthesis of spherical-like Pt-
MCM-41 meso-materials with high catalytic
performance for hydrogenation of nitrobenzene’,
Journal of Colloid and Interface Science.
Elsevier Inc., 346(2), pp. 486–493. doi:
10.1016/j.jcis.2010.03.018.
‘No Title’ (2011), 08(03), pp. 71–79.
Park, Y. et al. (2006) ‘Encapsulation method for the
dispersion of NiO onto ordered mesoporous
silica, SBA-15, using polyethylene oxide (PEO)’,
Journal of Colloid and Interface Science, 295(2),
pp. 464–471. doi: 10.1016/j.jcis.2005.09.006.
Schubert, Ulrich S. and Husing, N. (2005) Synthesis
of Inorganic Materials. 2nd, Revis edn. German:
Wiley-VCH.
Shah, A. T., Li, B. and Ali Abdalla, Z. E. (2009)
‘Direct synthesis of Ti-containing SBA-16-type
mesoporous material by the evaporation-induced
self-assembly method and its catalytic
performance for oxidative desulfurization’,
Journal of Colloid and Interface Science.
Elsevier Inc., 336(2), pp. 707–711. doi:
10.1016/j.jcis.2009.04.026.
Slowing, I. I. et al. (2008) ‘Mesoporous silica
nanoparticles as controlled release drug delivery
and gene transfection carriers’, Advanced Drug
Delivery Reviews, 60(11), pp. 1278–1288. doi:
10.1016/j.addr.2008.03.012.
Smith, A. L. (1960) ‘Infrared spectra-structure
correlations for organosilicon compounds’,
Spectrochimica Acta, 16(1–2), pp. 87–105. doi:
10.1016/0371-1951(60)80074-4.
Yan, Z. et al. (2006) ‘Pyridine-functionalized
mesoporous silica as an efficient adsorbent for
the removal of acid dyestuffs’, Journal of
Materials Chemistry, 16(18), pp. 1717–1725.
doi: 10.1039/b517017f.
Zhao, Q. et al. (2011) ‘Stability and textural
properties of cobalt incorporated MCM-48
mesoporous molecular sieve’, Applied Surface
Science. Elsevier B.V., 257(7), pp. 2436–2442.
doi: 10.1016/j.apsusc.2010.09.114.
Magnesium Impregnated Silica Mesoporous Prepared using Ester Ricinoleic as Template for the Esterification
279
