
Optimization of Polymer Networks using Variations in Moleculer
Weight of Polyetilen Glycol in the Manufacture of Semi – IPN
Hidrogels from Coconut Water Cellulose Bacteria
Tamrin* and Emma Zaidar
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara
Keywords: Hydrogel IPN, Coconut Water, Bacterial Cellulose.
Abstract: Research heve been done to find out the effect of variation moleculer weight polietylen glikoln 1000; 3000
and 6000 on manufacture semi interpenetrating polymer networks hidrogel from bacterial selulose with
sytem fermentation using accetobacterial-xylinum in based coconut water. IPN hidrogel by polietilenglikol
6000 has more croslinks compared using peg 3000 and peg 1000. Analysis thermal hidrogel IPN using peg
6000 is 457,14
0
C, compared PEG 3000 is 252, 80
0
C and PEG 1000 is 249,30
0
C. semi-IPN hydrogel
formed was characterized by, crosslink percentage, chemistry structural analysis using Fourier Transform
Infrared (FT-IR) spectroscopy, water absorption test and thermal resistance using Differential Thermal
Analysis (DTA). From the analysis, they showed Semi Interpenetrating Polymer Network Hydrogel with
using PEG 6000 the highest crosslink percentage (64,3%) for PEG 3000 is 42.5 % and PEG 1000 is 15. 7
%. Characterization results of FTIR indicate the occurrence of crosslinking between Polyacrylic acid and
MBA. This shown in existence of 1403 cm
-1
and 1560 cm
-1
(COO
-
) and 3413 cm
-1
(NH amine). Thermal
analysis using Differential Thermal Analysis (DTA) shows thermal optimum thermal stability at the
addition of Polietilengiikol 6000 to semi-IPN hydrogel which was completely degraded at 680
o
C, reaction
that occurs during the decomposition process is endothermic and exothermic reaction.
1 INTRODUCTION
Hydrogel is a hydrophilic polymer with a network
structure that has crosslinking. Hydrogels have the
ability to absorb a certain amount of water without
the dissolution process and have hydrodynamic
properties of cells in many ways (Lee, 2009).
Making hydrogels can be done by several
methods, namely graft polymerization methods,
crosslinking physics, chemical crosslinking, and
crosslinking radiation (d). One way to improve the
mechanical properties of the hydrogel is to make
modifications through the formation of the
Interpenetration Polymer Network (IPN) (Rimmer,
2011).
Making semi-JPI hydrogels for biomedical
applications can be mixed with natural ingredients.
Another natural material that may be developed as
an alternative to biomedicine is Centella asiatica
plant. Gotu Kola is believed to cure various types of
diseases because it has bioactive components that
are useful for the body. The chemical content of
gotu kola including Triterpenoid: asiaticosida,
madekasosida, cyanic acid, indosentoat acid,
bayogenin; Flavonoids: kaemferol, kuesertin;
Saponins: sentelasapogenol A, B and D; and Tanin
(BPOM RI, 2010). Bioactive components contained
in gotu kola have a function for health, one of which
is antibacterial. Bioactive components of gotu kola
which have antibacterial properties are flavonoids,
tannins and saponins (Zhao et al., 2009). The
mechanism of flavonoids inhibits bacterial growth
by denaturing bacterial cell proteins, causing all
bacterial cell metabolism to stop, tannins can inhibit
bacteria by changing the permeability of the
cytoplasmic membrane. Saponins can form complex
compounds with cell membranes through hydrogen
bonds so that they can destroy the permeability of
bacterial cell walls.
Zhao et al, (2009) stated that Pegagan ethanol
extract had higher antimicrobial activity than
petroleum ether and water extract. The results
showed that Gotu kola ethanol extract had a
minimum inhibitory content (MIC) of 125 µg / ml in
Propionibacterium vulgaris, Staphylococcus aureus,
Tamrin, . and Zaidar, E.
Optimization of Polymer Networks using Variations in Moleculer Weight of Polyetilen Glycol in the Manufacture of Semi – IPN Hidrogels from Coconut Water Cellulose Bacteria.
DOI: 10.5220/0009003203290334
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 329-334
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
329
Escherichia coli, Aspergillus niger and Candida
albicans. Whereas in Bacillus subtilis and
Aspergillus flavus is 62.5 µg / ml. (Heibesh et al.,
2013) found antimicrobial activity of asiatic acid
which is a saponin derivative in gotu kola against
several gram positive and gram negative bacteria.
Gotu kola is also used as a toothache medicine in the
general public, but until now there has been no
research or clinical data that supports it.
Heibesh (2013), has made a semi-JPI hydrogel
by mixing isopropyl acrylamide and cellulose. From
the results of Heibest's research, it was obtained
semi-JPI because cellulose was used in the form of a
linear carbon chain which was optimum at the
addition of cellulose by 20%.
Bajpai (2014), has made a semi-JPI hydrogel
from microcrystalline cellulose and acrylic acid with
N crosslinking, N'-Methylene Bisacrylamide.
Microcrystalline cellulose produced from cellulose
bacteria was dissolved in PEG (1000, 3000, and
6000) / NaOH solvents which were then
polymerized.
This study aims to make semi-JPI hydrogels
using various types of polyethylene glycol and
analyze how the crosslink degree and absorption
power obtained in PEG / NaOH solvent systems
with acrylic acid using N, N'-Methylene
Bisacrylamide crosslinking and the effect of adding
bacterial cellulose which functions as a biopolymer
which can improve the nature of hydrogel
absorption.
2 EXPERIMENTALS
Bacterial cellulose is made from coconut water with
the help of Acetobacter-xylinum bacteria. put into a
beaker glass containing 100 mL solvent system
Polyethylene Glycol 1000 (6% wt / vol) and NaOH
(8% wt / vol). The dispersion that occurred in the
refrigerator at -5
o
C for 24 hours. The frozen solid
obtained was left at room temperature with stirring
for 2 hours. The clear solution produced is then
filtered with ordinary filter paper which will be used
to make hydrogels. The same method is carried out
using 3000 polyethylene glycol and polyethylene
glycol 6000.
2.1 Hydrogel Preparation
A total of 5 mL of bacterial cellulose (BC) 1000
solution was put into a glass beaker then added
10.75 mmol of Acrylate Acid monomer, 200 µmol
of initiator of Potassium Per Sulfate, then added
little by little 260 µmol N crosslinking, N'-
Methylene bisacrylamide. Then stir for 15 minutes
at 60
o
C. After the polymerization is complete, the
results of the spring JPI hydrogel are poured into a
test tube, then heated into the oven at 60
o
C for 2
hours. After that, the results of the hydrogel were
released and flowed with distilled water and then
stored in a desiccator for 3 days.
Table 1: Comparison of Additions of BC 1000.
Sample
BC
(g)
Aac
(mmol)
MBA
(umol)
KPS
(umol)
A
0.2
10.75
260
200
B
0.4
C
0.6
D
0.8
E
1.0
Table 2: Comparison of Additions of BC 3000.
Sample
BC
(g)
Aac
(mmol)
MBA
(umol)
KPS
(umol)
A
0.2
10.75
260
200
B
0.4
C
0.6
D
0.8
E
1.0
Table 3: Comparison of Additions of BC 6000.
Sample
BC
(g)
Aac
(mmol)
MBA
(umol)
KPS
(umol)
A
0.2
10.75
260
200
B
0.4
C
0.6
D
0.8
E
1.0
2.2 Test of Water Absorption
Testing of water absorption was carried out by
determining the percent swelling ratio by measuring
the initial weight (mo) of the sample which was then
immersed in distilled water for 24 hours. The
samples that have been soaked are then filtered
using filter paper and measured again heavily (me).
The amount of water absorbed in the hydrogel can
be calculated using the following equation:
E = (me-mo) / mox 100% (1)
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
330

2.3 Percentage Test of Crosslinks
The percentage of crosslinking was carried out by
determining the crosslink percent percent where the
dry weight of the resulting hydrogel was weighed.
Then the hydrogel is soaked with a solvent
(chloroform) for 24 hours. After immersion, the
hydrogel is heated at a temperature of 60
o
C to dry
for 3 hours. The dry weight of the hydrogel after
immersion is determined by weighing using an
analytical balance. The degree of crosslinking can be
determined by the following equation:
% DC = Wg / (Wo) x 100 (2)
Where Wg is the weight of the dry hydrogel after
immersion and Wo is the weight of the dry hydrogel
before immersion.
2.4 FTIR Analysis
The specimens are clamped at the place where the
sample is then placed on the tool in the direction of
infrared light. The results will be recorded on a
paper scale of the wave number curve to the beam
intensity in the form of a spectrum graph.
2.5 TGA Analysis
Weighed ± 10 mg of sample, then put into aluminum
cell then pressed. The pressed cell is placed in a
position adjacent to the reference cell. After the
instrument is in equilibrium, the analysis device is
operated with a temperature of 40ºC to 600ºC with a
speed of heating increase of 10ºC / minute and the
gas used is nitrogen. The results obtained are in the
form of graphs of% of mass lost to temperature.
3 RESULTS AND DISCUSSIONS
Making semi-JPI hydrogels was obtained through
free radical polymerization of acrylic acid
monomers (AA) in the presence of MBA
crosslinkers and dissolved cellulose polymers. The
polymer network formed consists of crosslinked
covalent poly (acrylic acid) chains that are
physically entangled with cellulose and parts of PEG
macromolecules. The illustration of making a semi-
JPI hydrogel is shown in Figure 3.1. The
crosslinking used in this study is N, N'-Metilen
Bisakrilamida (MBA) which reacts with carboxyl
functional groups in the polymer chain so that a
polymer network is formed as shown in Figure 1 and
2.
Figure 1: Manufacture of Semi-IPN Hydrogel.
Figure 2: Polylactic acrylic crosslinking process.
Figure 3 showed the final form of the semi-IPN
Hydrogel.
Figure 3: Semi-IPN hydrogel with the addition of KMS as
much as (a) 0.2 g (b) 0.4 g (c) 0.6 g (d) 0.8 g (e) 1.0 g for
polyetilen glycol 6000.
Optimization of Polymer Networks using Variations in Moleculer Weight of Polyetilen Glycol in the Manufacture of Semi – IPN Hidrogels
from Coconut Water Cellulose Bacteria
331
The percent value of cellulose hydrogel swelling
ratio can be seen in Table 4
Table 4: Percentage of Semi-IPN Hydrogel Swelling Ratio
Data.
Weight
KMS
(g)
Initial
Weight
(g)
Final
Weight
(g)
Swelling
(%)
0.2
0.83
4.00
382
0.4
0.83
5.40
551
0.6
0.98
6.51
564
0.8
0.83
6.50
683
1.0
0.71
5.05
611
Based on Table 4, it is seen that the percent value
of the swelling ratio increases with increasing KMS
weight. This is because the amount of weight of
KMS in the polymerization mixture affects the
percent value of the swelling ratio in the hydrogel.
The more the number of KMS, the -OH group
group also increases, causing high hydrophilicity.
Therefore, the water in the hydrogel increases.
However, at the addition of 1.0 grams of KMS, the
percent swelling ratio decreases again, this is due to
the optimum physical interaction which causes the
diffusion of water in the hydrogel to decrease,
according to Bajpai's research (2014) that cellulose
hydrogels can absorb water due to the presence of -
OH group of cellulose.
Data determination of the degree of crosslinking
can be seen in Table 5.
Table 5: Data on the degree of crosslinking of Semi-JPI
Hydrogels.
Weight
KMS
(g)
Initial
Weight
(g)
Final
Weight
(g)
Crosslink
(%)
0.2
0.55
0.18
32.70
0.4
0.71
0.25
35.21
0.6
0.54
0.20
37.03
0.8
1.37
0.58
42.30
1.0
1.57
0.61
38.80
Based on Table 5, it can be seen that the percent
degree of crosslinking increases with the increase in
the number of KMS used. This is because the more
number of KMS used, the hydrogel will be more
dense but still elastic. Cross ties play an important
role in determining elasticity. The expected network
is the formation of chains as long as possible and
cross-bound only in a few places. On the addition of
KMS 1.0 gram percent crosslinking degree has
decreased. This is due to physical interactions or the
formation of hydrogen bonds between hydrogen
groups from KMS with hydrogen bonds from acrylic
acid, these bonds are like hydrogen bridges or
vanderwalls thus increasing the limited elasticity
properties. In this case the physical bond that occurs
has been optimum and so there is a decrease in
mechanical properties (addition of 1.0 gram KMS)
as shown in table 5. This decrease is due to the
occurrence of equilibrium so that the addition of
KMS can reduce mechanical properties due to
irregular polymer chain chains.
Figure 4: Commercial and Semi-IPN Hydrogel KMS
Spectrum.
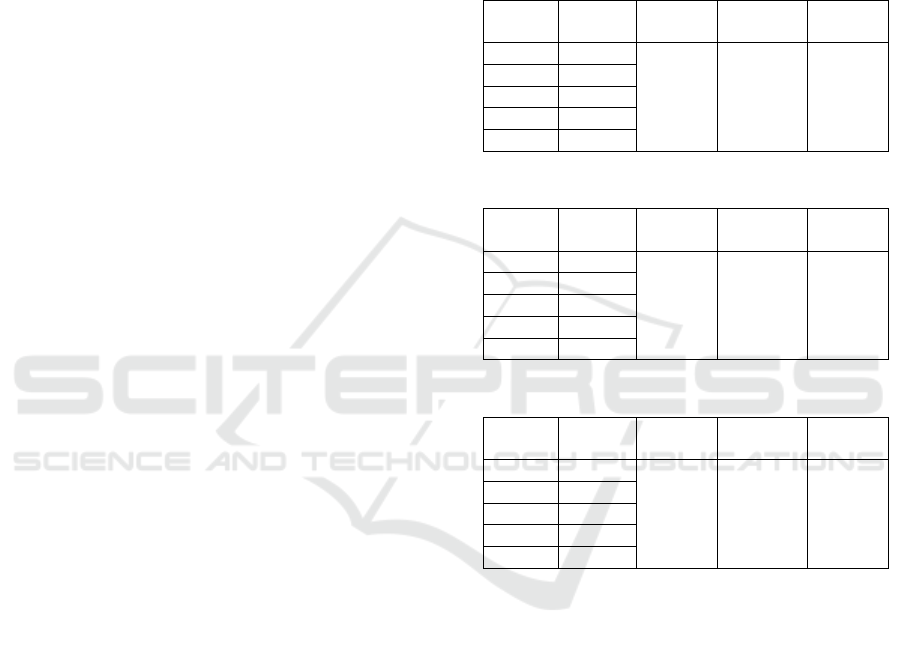
In the FTIR spectrum of commercial
carboxymethyl cellulose it was seen that the
absorption area of 1635 cm
-1
and 1427 cm
-1
showed
the presence of COO-carboxyl strain vibrations and
CH2 bonds originating from the ester group. This
shows the characteristic of carboxymethyl cellulose
compounds containing carboxyl groups as a
substitution between monochloro acetate and
cellulose compounds. So based on the results of the
FTIR analysis it can be concluded that true
commercial carboxymethyl cellulose is a
carboxymethyl cellulose compound.
In the FTIR spectrum of Semi-JPI Cellulose
Hydrogel it was seen that the absorption area at
wave number 1403 cm
-1
and 1560 cm
-1
showed
stretching by Karaaslan simetry and asymmetry of
carboxylic anions (2001). In the absorption area with
wave numbers 1636 cm
-1
and 2110 cm
-1
, the
presence of C = C and CH2 groups originating from
crosslinking. OH wave number 3600 cm
-1
to 2400
cm
-1
is OH group carboxylic acid, and OH group
absorption alcohol about 3400 cm
-1
(Sadeghi and
Yarahmadi, 2011). So that the absorption of the OH
group carboxylic acid covers uptake of OH KMS
groups and OH groups from PEG 1000 and NH
amide groups from the MBA.
DTA is used to study thermal properties and
phase changes due to enthalpy changes of a material.
DTA analysis has been performed on the optimum
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
332
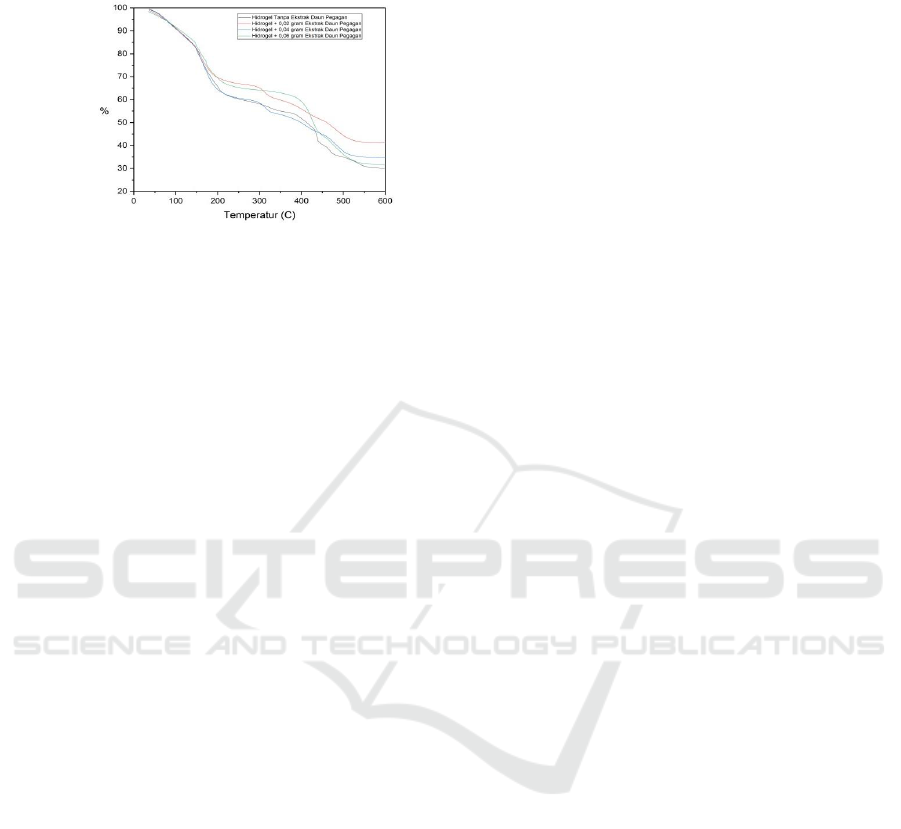
semi-JPI Hydrogel sample which can be seen in
Figure 5.
Figure 5: DTA results from optimum Semi-IPN
Hydrogels.
In Figure 5 shows the thermogram of DTA for
semi-JPI hydrogels with the addition of 0.8 grams
of KMS. DTA analysis is carried out at a
temperature of 20-650
o
C. The endothermic reaction
occurs at 120
o
C, the first exothermic point occurs at
380
o
C, and the second exothermic point occurs at
650
o
C which shows that the hydrogel has
completely degraded. Hydrogel with the addition of
0.8 gram KMS has the highest thermal stability
(optimum) this is directly proportional to the
crosslinking degree of the hydrogel, where the
higher the percent degree of crosslinking the
hydrogel has a high thermal resistance as well.
According to Bajpai (2014), hydrogels at
temperatures below 350
o
C of water molecules
bound to the polar group are released, and
decarboxylation of COO groups associated with
Poly (Sodium Acrylate) occurs. When the
temperature is above 350-433
o
C, the number of
PEG is low. At temperatures of 415-510
o
C, the loss
of CO and CO
2
groups with a small amount of each
other overlap. In the end, slowly and gradually at
temperatures above 510
o
C carbonization or
degradation of the process into ash.
4 CONCLUSIONS
1. The optimum mixture concentration in making
semi-JPI hydrogels between KMS and Acrylate
Acid in the presence of crosslinking N, N-
Methylene Bisacrylamide and initiator of
Potassium Per Sulphate is in comparison of
KMS: KPS: MBA (0.8: 0.05: 0.041 b / b) where
as much as 0.8 gram KMS is dissolved into the
PEG 1000 / NaOH solvent system which is then
added to 0.05 grams of KPS initiator and 0.041
grams of the MBA crosslinker.
2. Effect of variation molecular polyetylen glycoln
weight 1000; 3000 and 6000 on manufacture
semi interpenetrating polymer networks
hydrogel from bacterial cellulose with
fermentation systems using accetobacterial-
xylinum in based coconut water. IPN hydrogel
by polyethylene glycol 6000 has more croslinks
compared to peg 3000 and peg 1000. Analysis
of IPN thermal hydrogel using peg 6000 is
457.14
o
C, compared to PEG 3000 is 252, 80
o
C
and PEG 1000 is 249.30
o
C. Semi-IPN hydrogel
formed was characterized by, crosslink
percentage, chemistry structural analysis using
Fourier Transform Infrared (FT-IR)
spectroscopy, water absorption test and thermal
resistance using Differential Thermal Analysis
(DTA). From the analysis, they showed the
highest Semi Interpenetrating Polymer Network
Hydrogel with polyethylene glycol 6000
crosslink percentage (64.3%) for crosslink
polyethylene glycol 3000 is 42.5% and
polyethylene glycol 1000 is 15. 7%.
Characterization results of FTIR indicate the
occurrence of crosslinking between Polyacrylic
acid and MBA. This is shown in existence of
1403 cm-1 and 1560 cm
-1
(COO-) and 3413 cm
-
1
(NH amine). Thermal analysis using
Differential Thermal Analysis (DTA) shows
that it was completely degraded at 680
o
C, the
reaction that occurs during the decomposition of
the process is endothermic and exothermic
reaction.
REFERENCES
Lee, J. 2009. Current Situation and Aplication Of Medical
Polymeric Materials.
http://polymerization.asia/articles.
Rimmer, S. 2011. Biochemistry, Manufacture and Medical
Applications. Biomedical Hydrogels. UK: Woodhead
Publishing
Zhao, S., Cao, M., Li, H., Xu, W. 2009. Synthesis and
Characterization Of Thermo-Sensitive Semi-IPN
Hydrogels Based On Poly (erhylene glycol) – co –
poly (Ɛ-caprolactore) macromer, N-
isopropylacrulamide, and Sodium Alginate.
Carbohydrate Research 345 (2010): 425-431
Heibesh, A., S, Farag., S, Sharaf., Th, I. Shaheen. 2013.
Thermal Responsive Hydrogels Based On Semi
Interpenetrating Network Of Poly (NIPAm) and
Cellulose Naniwhiskers. Carbohydrate Polymers. 102
(2014) 159-166
Bajpai, S. K. 2014. New Semi-IPN Hydrogels Based on
Cellulose for Biomedical Application. Journal Of
Polymer. India: Hindawi Publishing Coorporation
Optimization of Polymer Networks using Variations in Moleculer Weight of Polyetilen Glycol in the Manufacture of Semi – IPN Hidrogels
from Coconut Water Cellulose Bacteria
333

Karaaslan MA, Tshabalala MA and Buschle-Diller G,
2010. Wood Hemicellulose / Chitosan – Based – Semi
– Interpenetrating Network Hydrogels: Mechanical,
Swelling, and Controlled Drug Release Properties.
BioResource 5 (2): 1036-1054Kirk Othmer. 2001.
Encyclopedia Of Chemical Technology.John Willey &
Sons.
Sadeghi M dan Yarahmadi M, 2011. Synthesis and
characterization of superabsorbent hydrogel based on
chitosan-g-poly (acrylic acid-co-acrylonitrile). African
Journal of Biotechnology, 12265-12275.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
334
