
Emulsion Treatment using Local Demulsifier from Palm Oil
Emre Fathan and Tomi Erfando
Departement of Petroleum Engineering, Universitas Islam Riau, Pekanbaru, Indonesia
Keywords:
Emulsion, Local Demulsifier, Palm Oil, Bottle Test
Abstract:
Conventional demulsifier (chemical) are still used until now in many of oil industries which the formulas are
both expensive and harmful for the environment.In this research, the new formula of local demulsifier will be
tested with palm oil, lemon, glycerin, and KOH as the materials. Those materials are more friendly for the
environment and contain hexane group and octadecenoic acid that are composition in plant that can break the
emulsion. Crude oil (20.8
◦
API) is taken from wellhead of the X Field in Riau, Indonesia. Emulsion sample
will be treated with formula local demulsifier and tasted in water bath for 3 hours vulnerable with 30 minutes
of observation. Bottle test method will be used with the following of 40
◦
C, 60
◦
C, and 80
◦
C as temperature
test.The test revealed that the formula demulsifier + lemon (DKL) given the best result than conventional
demulsifier within 120 – 180 minutes at 80
◦
C that separated 39 ml of water with 5 ml of concentration.P-value
of temperature is the only less than the significance value (α=0.05) means that the linear regression model meet
the criteria of linearity and the changes that occur are significant.
1 INTRODUCTION
The participation of water in the production process of
oil is common in upstream oil and gas activities. The
water is formation water that has a chemical content
that will cause problems in the series of equipment
both under and above the surface. The occurrence
of one of them is the forming of an emulsion.
These problems result in high pumping costs, pipe
corrosion, and special handling of certain equipment
(Abdel-Raouf, ). An emulsion is a mixture of two
immiscible fluids, one of which is shaped droplets
on the other and chemically bound or stabilized
by emulsifying agents (Soffian and Niven, 1993).
Demulsifier injection is often used to overcome
emulsion problems. The process of breaking down
oil-water emulsions into an oil phase and the water
phase is called the demulsification (Kokal, 2005).
However, its use is still using commercial
(chemical) materials which are relatively expensive
(Emuchay, Onyekonwu, Ogolo, & Ubani, 2013)
and cause damage to the environment. In several
studies, demulsifier tests with local materials have
been carried out, for example testing with coconut
oil (Emuchay et al., 2013),lime(Erfando et al., 2018),
and curcas oil (Sulaiman et al., 2015). Where in
all the three studies shows the potential of local
demulsifiers.The potential in the oil and gas sector
should be developed to increase local and national
revenues(Erfando and Herawati, 2017).
In this study, new of local demulsifier are
formulated to minimize the negative impact of
commercial demulsifier both in reduce the high
cost and minimize the negative impact of using
chemical on the environment. The new local
demulsifier formula will be formulated using palm
oil, gliserin, lemons and KOH compounds. Palm oil
contain hexane group and octadecenoic acid. Those
compositions are two main plant components that can
break the emulsion (Yaakob and Sulaimon, 2017).
For the result, those local and commercial
demulsifiers will be comparing within take abest
result of temperature, concentration of separation,
and the time of separation.This study was conducted
to know which formula will give the best result in
separated the water and to know the contribution of
the parameter toward the test through analysis of
regression.
The emulsion is defined as a colloidal system
in which small grains from one of the phase
presses in other phases where they are usually not
mutually mixed. An emulsion can be found in the
production process and equipment. The type that
we often encounter in the field is water emulsion
in oil (w/o). The stability of the emulsion itself
cannot be separated from crude oil asphaltenes
Erfando, T. and Fathan, E.
Emulsion Treatment using Local Demulsifier from Palm Oil.
DOI: 10.5220/0009360102990303
In Proceedings of the Second International Conference on Science, Engineering and Technology (ICoSET 2019), pages 299-303
ISBN: 978-989-758-463-3
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
299

and resins (Abdel-Raouf, 2012). Emulsion in the
production field can be classified into three types,
Water-Emulsion in Oil (W/O);Oil Emulsion in Water
(O/W); and Complex Emulsion (Multiple/Complex).
Emulsion is an unstable system, according to (Wylde
et al., 2009), classifying the length of time an
emulsion system is separated based on its stability:
• Loose emulsion: is separated in minutes;
• Medium emulsion: separate in 10 minutes or
more;
• The emulsion sight will be stable for hours or even
days and in some cases, will not be able to be
overcome.
Some of the causes of the stability of the
emulsion are explained in the study (Kokal, 2005).
Such as agitation, the grain size, surfactant, effect
of pH, the composition of brine water, viscosity,
and temperature. For demulsification of emulsion,
injecting the demulsifire is one of the option for
separate the emulsion to dissolve dispersed phase
from the dispersing phase. The molecule of a
demulsifier will mobilize to the interface of oil-water
and separated both natural surfactant (asphaltenes
and resins) (Zhou et al., 2014). Over the years
there has been an over dependence on the use of
foreign/commercial demulsifiers this has been found
not to be quite effective in most cases due to
incompatibility with the nature of some kinds of
crude (Emuchay et al., 2013). For some cases it
will led to the challenge for the advanced studies to
locallyformulated demulsifier in result for improved
cost efficiency and effectiveness.
2 MATERIAL & METHOD
2.1 Material
The material we used for laboratory study of emulsion
and demulsification are a water bath (Memmert
WNB 14), heater and stirrer (Wisd.), digital scale
(Amastech), bottle for test (duran), several breakers
(Pyrex Iwaki TE-32), several graduated cylinder
(Pyrex), density bottle, and stopwatch. For producing
local demulsifier we used a commercial palm
oil, potassium hydroxide (KOH), aquades, glycerin
(C
3
H
8
O
3
), and citrus limon.
2.2 Method
Generally we used saponification, bottle test method,
and statistical test. In order to produce the liquid soap
as a base of local demulsifier, we used saponification
with following step based on (Naomi et al., 2013;
Sukeksi et al., 2017; Zulkifli and Estiasih, 2014).
Bottle test used to observing the result and converting
the data into graphic. As for statistical method will
be using statistic application that allows providing
which parameter (time of separation, temperature, or
injecting volume) most contribute for the test.
2.2.1 Production of Local Demulsifier
Local demulsifier (DKS) will be formulated with
saponification method, with the following step;a) 50
ml of palm oil commercial was added to a breaker
and heated with 80
◦
C for 30 minutes. b) 12.5 g of
KOH add into breaker along with 25 ml aquades and
then heated until homogenous; c) Add KOH + Aq into
palm oil and stir it with heater and stirrer in 80
◦
C, 800
rpm, for 3 hours and 20 minutes; d) For the last add
50 ml of aquades and stir for 5 minutes, then wait
the formula for 24 hours until the formula become
liquid. Both formulas are the local demulsifier for this
research.
2.2.2 Demulsification with Bottle Test Method
The following formulas that will be tasted are: a)
Local demulsifier (DKS); b) Local demulsifier +
lemon (DKL); c) Commercial demulsifier (DK);and
d) Base case (without adding demulsifier). Those
formulas will be injected to a bottle of sample
emulsion (1 ml, 3 ml, and 5 ml). Each volume are
tested in several temperature (40
◦
C, 60
◦
C, and 80
◦
C)
for 3 hours.
Emulsion separation was recorded at various time
intervals (Hirasaki et al., 2010).The process was
monitored for every 30 minutes in 3 hours. The step
based on (Erfando et al., 2019; Hirasaki et al., 2010)
3 RESULT
Table 1 is the properties data for sample oil. The
data was calculate to determine the type of oil. The
type of oil sample is heavy oil with SG = 0.929 and
20.8
◦
API. Figure 1-3 are the result of the test with
bottle test method in water bath.From those figures
we found out the best, highest, and also the bad
separation within the formulas.
Based on data, base case formula has the highest
separation in figures 1 (40
◦
C). Meanwhile not the
case in temperature of 60
◦
C and 80
◦
C. Formula
DKShas the highest separation value on figure 2 when
adding 5 ml concentration. Figures 2 shown that
ICoSET 2019 - The Second International Conference on Science, Engineering and Technology
300

commercial formula (DK) has stable separation at
temperature of 60
◦
C. Meanwhile bad at temperature
of 40
◦
C (figure 1).
Table 1: Properties of Crude Oil
No Properties Value Unit
1 Oil Mass 23.2 gr
2 Oil
Density
0.93 gr/ml
3 Specific
Gravity
0.93 -
4
◦
API 20.8 -
In figure3, formula DKL (5 ml,80
◦
C) given the
stable and highest separation from 120 to 180 minutes
with temperature of 80
◦
C.The result from the bottle
test method shown in figure 3, that the value of local
+ lemon demulsifier (DKL) separation is 39 ml.
Based on the data above, the best result
shown in figure 3 as DKS and it takes 120 –
180 minute for water separated from emulsion
sample.(Hayuningwang et al., 2015) said, moreover
the salinity and temperature here also affects the
amount of separation of water, but the higher the value
of salinity the process of separating oil from water
takes longer.
Based on data, the additional of 5 ml is the best
concentration for injecting the formulas into sample,
while temperature of 80
◦
C is the ideal temperature
in this research.(Augustina and Sylvester, 2015)said,
the temperature or heat broke up some of the weak
emulsion thereby causing coalescence and dropping
of water out of the emulsion which settle in the bottom
of bottle. When the temperature is rise there is also an
increase of demulsification efficiency. The research
of (Erfando et al., 2019) also make an explanation
that the temperature is one of the parameters that can
affect the condition of emulsion significantly.
3.1 Analysis of Regression and
Correlation
Table 2: Regression Analysis Data
No Parameter P-Value R-Sq
R-Sq
(adj)
1 Temperature 0 62.2 62.1
2 Injected Volume 0.362 0.4 0.1
3 Time 0.1 0.9 0.6
Comparison of the linear regression models
determines the effect of variables X on Y (Subekti,
2015). If the contribution is positive then the
value of variable X agrees to the value of variable
Y.Theanalysis of regression and correlation from this
research are from statistical software, to get the
information of regression and correlation from the
parameters (time, injected volume, and temperature)
versus separation.
From table 2, at the output obtained p-valueof
temperature is the onlyless than the significance value
(α=0.05) means that the linear regression model meet
the criteria of linearity and the changes that occur are
significant(Draper and Smith, 1998).
R-Sq (ad j) of temperature is 62.1%, the value is
interpreted as a percentage of contribution in the test.
Both parameter injected volume and time have each
0.1% and 0.6%. From those data temperature has the
highest contribute.
4 CONCLUSION
Based on laboratory test, formula DKL given the
high result than conventional demulsifier within 120
– 180 minutes at 80
◦
C that separated 39 ml of water
with 5 ml of injected volume. The effectiveness of
emulsion breakdown using local material is better
based on base case reference and it’s comparison with
a conventional demulsifier result.The temperature has
the biggest contributes among all the parameters seen
from the regression analysis data.
ACKNOWLEDGMENTS
The author gratefully acknowledge financial
support from Universitas Islam Riau and Petroleum
Engineering’s laboratory for the facilities.
Emulsion Treatment using Local Demulsifier from Palm Oil
301
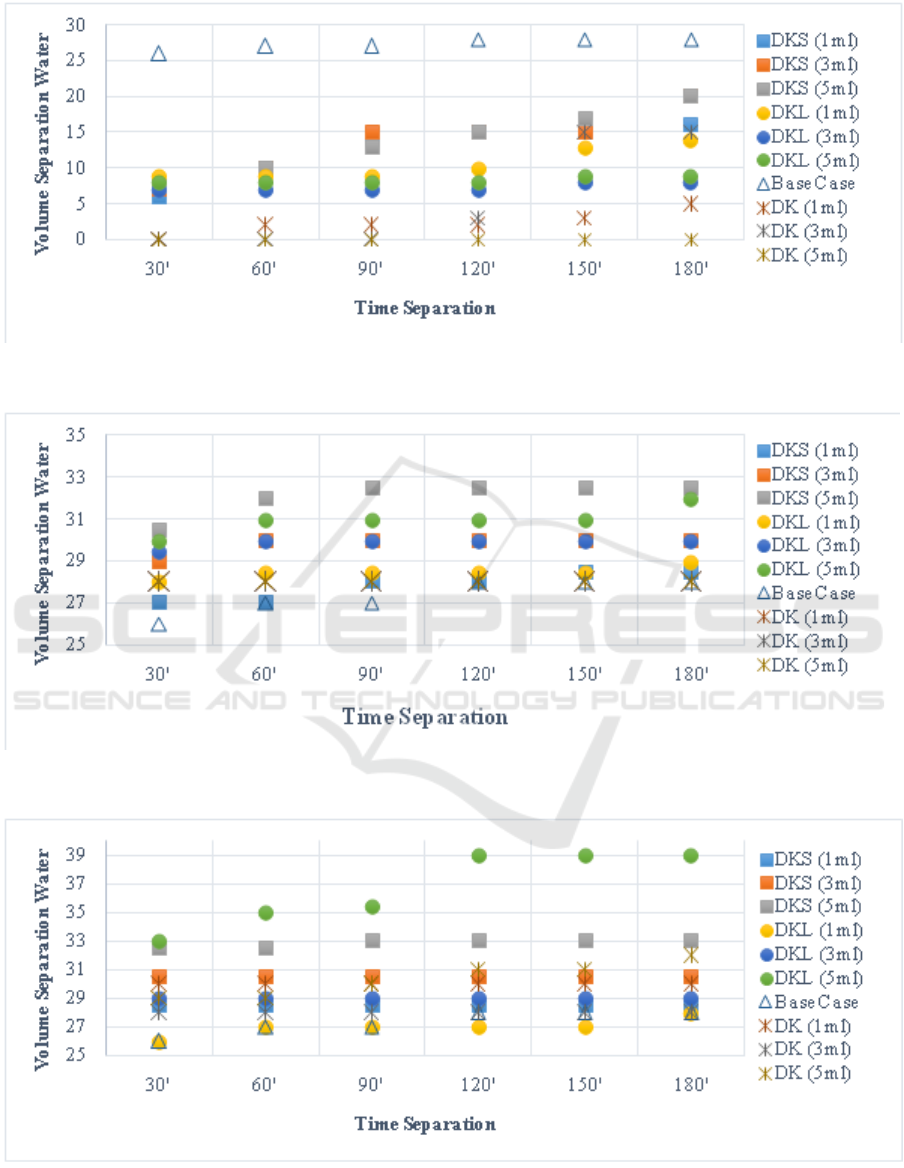
Figure 1: Bottle test in temperature of 40
◦
C.
Figure 2: Bottle test in temperature of 60
◦
C.
Figure 3: Bottle test in temperature of 80
◦
C.
ICoSET 2019 - The Second International Conference on Science, Engineering and Technology
302

REFERENCES
Abdel-Raouf, M. E. S. (2012). Factors affecting the stability
of crude oil emulsions.
Augustina, O. and Sylvester, O. (2015). Emulsion
Treatment in the Oil Industry: A Case Study of Oredo
Field Crude Oil Emulsion. SPE Nigeria Annual
International Conference and Exhibition.
Draper, N. R. and Smith, H. (1998). Applied regression
analysis (Vol. 326).
Emuchay, D., Onyekonwu, M. O., Ogolo, N. A., and
Ubani, C. (2013). Breaking of emulsions using
locally formulated demusifiers. SPE Nigeria Annual
International Conference and Exhibition.
Erfando, T., Cahyani, S. R., and Rita, N. (2019). The
utilization of citrus hystrix and citrus limon as an
organic demulsifier formulation. IOP Conference
Series: Materials Science and Engineering,
509(1):12145.
Erfando, T. and Herawati, I. (2017). Analysis of petroleum
downstream industry potential in riau province.
Journal of Geoscience, Engineering, Environment,
and Technology, 2(2).
Erfando, T., Rita, N., and Cahyani, S. R. (2018).
Identifikasi Potensi Jeruk Purut Sebagai Demulsifier
Untuk Memisahkan Air Dari Emulsi Minyak di
Lapangan Minyak Riau. Jurnal Kimia Mulawarman,
15(2).
Hayuningwang, D., Fadli, A., and Akbar, F. (2015).
Pengaruh Salinitas KCl & NaCl terhadap Kestabilan
Emulsi Minyak Mentah–air di Lapangan Bekasap. PT.
Chevron Pacific Indonesia. Jurnal Online Mahasiswa
Fakultas Teknik Universitas Riau, 2(1).
Hirasaki, G. J., Miller, C. A., Raney, O. G., Poindexter,
M. K., Nguyen, D. T., and Hera, J. (2010). Separation
of produced emulsions from surfactant enhanced oil
recovery processes. Energy & Fuels, 25(2).
Kokal, S. L. (2005). Crude oil emulsions: A state-of-the-art
review. SPE Production & Facilities.
Naomi, P., Gaol, A. M. L., and Toha, M. Y. (2013).
Pembuatan sabun lunak dari minyak goreng bekas
ditinjau dari kinetika reaksi kimia. Jurnal Teknik
Kimia, 19(2).
Soffian, R. M. and Niven, T. L. (1993). Emulsion Treatment
Program. SPE Asia Pacific Oil and Gas Conference.
Subekti, P. (2015). Perbandingan Perhitungan Matematis
Dan SPSS Analisis Regresi Linear Studi Kasus
(Pengaruh IQ Mahasiswa Terhadap IPK). 1–21.
Sukeksi, L., Sidabutar, A. J., and Sitorus, C. (2017).
Pembuatan Sabun dengan Menggunakan Kulit Buah
Kapuk (Ceiba Petandra) sebagai Sumber Alkali.
Jurnal Teknik Kimia USU, 6(3).
Sulaiman, A. D. I., Abdulsalam, S., and Francis, A. O.
(2015). Formulation of Demulsifiers from Locally
Sourced Raw Materials for Treatment of a Typical
Nigerian Crude Oil Emulsion. (January 2015).
Wylde, J. J., Coscio, S. E., and Barbu, V. (2009). A case
history of heavy-oil separation in northern alberta:
A singular challenge of demulsifier optimization and
application. SPE Production & Operations.
Yaakob, A. B. and Sulaimon, A. A. (2017). Performance
assessment of plant extracts as green demulsifiers.
Journal of the Japan Petroleum Institute, 60(4).
Zhou, H., Dismuke, K. I., Lett, N. L., and Penny,
G. S. (2014). Development of More Environmentally
Friendly Demulsifiers. (February), 15–17.
Zulkifli, M. and Estiasih, T. (2014). Sabun Dari Distilat
Asam Lemak Minyak Sawit: Kajian Pustaka [In Press
Oktober 2014]. Jurnal Pangan Dan Agroindustri,
2(4).
Emulsion Treatment using Local Demulsifier from Palm Oil
303
