
Mass Transfer Modeling of Acid Violet 17 Adsorption onto Activated
Carbon
Puguh Setyopratomo, Hadiatni Rita Priyantini and Rudy Agustriyanto
Chemical Engineering Department, Faculty of Engineering, University of Surabaya, Raya Kalirungkut, Surabaya 60293
Keywords: Acid violet 17, Activated carbon, Dyes adsorption, Kinetic model.
Abstract: This study was conducted to determine the suitability of the observed equilibrium and kinetic model, as well
as the removal of acid violet 17. The batch experimental variables include the dyes initial concentration and
the ratio of the adsorbent mass to the volume of the solution. The adsorption equilibrium data were examined
using three equilibrium models i.e., Linear, Freundlich, and Langmuir. The observed model is limited by two
forms of the proposed kinetic model. The first model just considers the convection mass transfer of the dyes
to the external surface of the adsorbent, while the second model also takes into account the solute diffusion
in particles. The experiment result showed that the adsorption process follows the Langmuir correlation with
the adsorption capacity of 185.19 mg/g. From this study, it was found that the adsorption is controlled by the
diffusion stage inside the particle. The dyes removal reach 58%, which was achieved when 0.0015-gram
adsorbent was used per ml solution. The results of this study can contribute to the design of the commercial
scale adsorption process.
1 INTRODUCTION
In recent years, the production of dyes continues to
grow in line with the growth of the industries using
dyes as coloring agents such as paper, textile,
cosmetic, and leather tanning (Jain & Gogate, 2017).
At present, approximately 10 thousand dyestuff
products on the market (Goswami & Phukan, 2017).
The chemical structure of dyes is a complex aromatic
molecule. Besides, they contain various functional
groups. This causes toxic and non-biodegradable
properties (Yu et al., 2019). They are very stable, so
they tend to accumulate in nature. Globally, the
annual consumption of dyes in the chemical industry
reaches 7x105 tons, and it was estimated that 10-15%
of which was disposed into the water stream, causing
serious environmental pollution (Gamoudi & Srasra,
2019), (Belbel et al., 2018).
Acid violet 17 is the anionic azo dyes and is
usually used as a coloring agent for textile and leather
(Saleem, Pirzada & Qadeer, 2007). The biggest
source of dye contamination in the water stream is the
textile industry (Li, Mu & Yang, 2019). Disposal of
the dyes from industries to the water stream will cause
many environmental problems. The presence of dyes
in the water, even at low quantity, is undesirable. It
will decrease the beauty of the surroundings and
threatens human health and the environment
(Boudechiche et al., 2019). Besides, its presence in
the water body will reduce the penetration of light
needed for photosynthesis of aquatic biota (Daoud et
al., 2019).
It was reported that the intake of organic dyes into
the body could cause damage to several important
organs such as the liver, digestive system, and
nervous system of human beings (Gao et al., 2019).
Other harmful effects of dyes are carcinogenic,
mutagenic, and cause kidneys dysfunction (Abd-
Elhamid et al., 2019). Therefore, the removal of dyes
from the flow of industrial wastewater before being
discharged into a water body is a must.
There are several processing methods available to
eliminate dyes from wastewater, such as adsorption,
photocatalytic degradation, biodegradation,
coagulation-flocculation, ion exchange, membrane
technology, and electrochemical oxidation (Liu et al.,
2019). Among the processing methods for dyes
removal from liquid waste, adsorption is still
considered to be a more efficient and inexpensive
technology because the process is simple, operated at
ambient temperature and pressure, and cost-efficient
(Thinakaran et al., 2008), (Khorasani &
Shojaosadati, 2019).
Setyopratomo, P., Priyantini, H. and Agustriyanto, R.
Mass Transfer Modeling of Acid Violet 17 Adsorption onto Activated Carbon.
DOI: 10.5220/0009423701290136
In Proceedings of the 1st International Conference on Industrial Technology (ICONIT 2019), pages 129-136
ISBN: 978-989-758-434-3
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
129

Various adsorbents are available to remove dyes
from aqueous solutions such as clay, activated
carbon, clay, zeolite, carbon nanotubes, graphene
oxide, polymeric materials, and agricultural waste
(Rajabi, Mahanpoor & Moradi, 2019), (Vahdati-
Khajeh et al., 2019). In practice, the most widely used
adsorbent for removing dyes from aqueous solutions
is activated carbon (Ferreira et al., 2017). Besides
having high efficiency, activated carbon has an
economic advantage because it can be prepared from
agricultural waste materials that are rich in cellulose
and lignin (Herrera-González, Caldera-Villalobos &
Peláez-Cid, 2019).
Adsorption is one of the most important
separation technologies, where the dissolved
compound is selectively attracted to the surface of the
adsorbent through the mass transfer process resulting
in the accumulation of the compound at the surface or
the interphase of the adsorbent. Adsorption can occur
physically due to physical force such as hydrogen
bonding, polarity, Vander Waals forces, and dipole-
dipole interactions, etc., or chemically due to the
exchange of electrons, where adsorbate is chemically
bonded to the surface of the adsorbent (Kausar et al.,
2018). In the phenomenon of adsorption, the
interaction between solute and adsorbent is very
specific, and this depends on the mutual interactions
which are influenced by their physical and chemical
properties (Benabi et al., 2019).
In general, the mechanism of solute adsorption
from an aqueous solution to the surface of a solid
adsorbent consists of the following three successive
stages, i.e., 1) external mass transfer, where the solute
moves from the bulk solution to the outer surface of
the adsorbent particle; 2) intraparticle diffusion (pore
volume diffusion, surface diffusion, or a combination
of both mechanisms); 3) adsorption of the solute
molecules on an active site of the adsorbent
(Ocampo-Perez et al., 2010).
The adsorption reaction model, which is one of
the most widely used to represent dyes adsorption
kinetic onto activated carbon and includes pseudo-
first-order and pseudo-second-order, ignores both
external mass transfer and intraparticle diffusion
resistance. (Dotto, Buriol & Pinto, 2014). Thus this
model assumes that the adsorption kinetic is fully
controlled by the rate of adsorption of solutes on the
surface of the adsorbent. Therefore, this model
expressed the rate of adsorption as identical to the rate
of a chemical reaction (Ocampo-Pérez et al., 2012).
The consequence of using this model is that neither
the mass transfer coefficient nor the rate-controlling-
stage can be obtained. However, to obtain a design for
a reliable commercial-scale adsorption process
system, detailed and accurate information regarding
mass transfer resistance and the stage which controls
the adsorption rate are required. Therefore, in this
study, adsorption kinetic models will be developed to
determine the external as well as the intra-particle
diffusion mass transfer coefficient of acid violet 17
adsorptions onto activated carbon. To solve such an
adsorption kinetic model, the adsorption equilibrium
correlation at the interface between the solid phase
and the liquid phase is required. Hence the objective
of this study is 1) to determine the correlation of
equilibrium adsorption of acid violet 17 onto
activated carbon; 2) to determine the external and the
intra-particle diffusion mass transfer coefficients; 3)
to investigate the percent dyes removal.
2 THEORY AND EXPERIMENT
2.1 Equilibrium Adsorption
Correlation
Equilibrium adsorption correlation is the relationship
between solute concentration in the liquid phase and
solid phase at equilibrium at a certain temperature.
Three adsorption equilibrium models will be
investigated for their suitability with the adsorption
system studied in this study, namely: the Langmuir
model, the Freundlich model, and the Linear model.
The Langmuir equilibrium model is expressed in
Equation (1). The Langmuir model assumes: (1) the
adsorbent has a homogeneous active side and can
only adsorb one layer of the adsorbate molecule, (2)
there is no interaction between the absorbed
molecules, (3) the same mechanism occurs in each
adsorption process. The Freundlich model is stated in
equation (2). The Freundlich model assumes that the
adsorbent has a heterogeneous surface, and each
molecule has different absorption potential.
Meanwhile, related to Henry's Law, equilibrium in
solid-liquid systems can be described by linear
equilibrium. This linear equilibrium is limited to low
equilibrium concentrations, i.e., for solute
concentrations below 50 ppm. The formulation of
linear equilibrium is expressed in Equation (3).
(1)
C
1
kC
1
C
1
C
1
eqmaxμ,maxμ,eqμ,
(2) KCC
1/n
eqeqμ,
(3) C HC
eqμ,eq
where, Cμ, eq: the mass of solute adsorbed per mass
of the adsorbent at equilibrium, Cμ, max: the
ICONIT 2019 - International Conference on Industrial Technology
130

theoretical monolayer saturation capacity, Ceq: solute
concentration in solution at equilibrium, k, K, n:
constant, H: a distribution constant.
2.2 Kinetic Model
In designing a system of adsorption processes, the
analysis of adsorption kinetic is a very important
stage. From the kinetic analysis, information about
the time needed to achieve the adsorption equilibrium
will be obtained. Furthermore, from the kinetic
analysis, the adsorption mechanism will be known
(Magdy & Altaher, 2018). Adsorption of solute in
porous particles will involve the following stages i.e.,
external mass transport, intra-particle diffusion, and
adsorption on an active site inside the pores.
(Ocampo-Perez et al., 2011). In this work, kinetic
models were developed on the basis of the following
general assumptions: (i) intraparticle diffusion occurs
by pore volume diffusion mechanism that follows
Fick's law of diffusion, (ii) adsorption reaction at the
surface of the adsorbent occurs instantaneously, (iii)
as an adsorbent, the activated carbon particles have a
spherical shape, (iv) within the adsorbent particles the
diffusion occurs only in the radial direction, (v)
adsorption takes place under isothermal conditions.
2.2.1 The First Kinetic Model
In the first kinetic model, solute adsorption is
considered to only occur from the bulk of the liquid
to the outer surface of the adsorbent. Thus there is no
gradient of solute concentration within the adsorbent
particles. At such condition, the rate of decreasing
mass of solute in the liquid phase is equal to the rate
of mass transfer of the solute from the bulk solution
to the outer surface of the adsorbent, and is
mathematically formulated in the following equation:
(4) CC
V
Ak
dt
dC
*
l
c
where: t: time, kc: the convection mass transfer
coefficient, A: the outer surface area of the adsorbent,
C: the solute concentration at the bulk of solution, C
*: the solute concentration at the liquid phase
interphase, Vl: total solution volume.
Meanwhile, the solute transfer rate from the
solution to the adsorbent is equal to the rate of solute
increase in the adsorbent, as stated in the following
equation.
(5) CC
m
Ak
dt
dC
*
p
c
μ
Where: Cμ: solute concentration in the adsorbent,
mp: the mass of adsorbent. The initial condition are:
at t=0, C = Co and Cμ = 0. The correlation between
the concentration in the solid phase and in the liquid
phase at the interface follows the Langmuir model.
This correlation acts as a boundary condition.
2.2.2 The Second Kinetic Model
The second kinetic model assumes that the adsorbent
is a porous medium so that the mass solute transfer
occurs in three stages, namely: (1) convection of the
solute from liquid bulk to the outer surface of the
adsorbent through the film layer on the outer surface
of the adsorbent, (2) intraparticle liquid phase
diffusion of solute from the outer surface of the
adsorbent to the active site of the adsorbent through
the pore, (3) attachment of the solute to the active site
of the adsorbent in the pore.
(6) ρCεC
t
Δrr 4
r
C
Δrr4πD
r
C
r 4D
adsμ
2
Δrr
2
e
r
2
e
(7)
t
C
23
4
r
C
2
4D
3
2
e
H
r
r
ads
(8)
r
C
R 4πD CCR 4πk
surface
2
e
*
LL
2
c
In the second model, it is assumed that diffusivity
and porosity are uniforms in all parts of the adsorbent.
While, as in the first model, the equilibrium between
the solute concentration in the liquid and solid phases
at the interface follows the Langmuir model. The
second model was developed by applying the
principles of solute mass balance in the particle and
manifested in three differential equations i.e. equation
(6) which applicable at the middle of the solid
adsorbent, equation (7) which applicable at the center
of the solid adsorbent and equation (8) which
applicable on the surface of the adsorbent, where, C:
concentration of solute in the liquid which fills the
adsorbent pore, ρads :density of the adsorbent, ε:
porosity of the adsorbent, R: adsorbent radius, r:
position in adsorbent in radial system, De: the
effective diffusivity of the solute, CL: solute
Mass Transfer Modeling of Acid Violet 17 Adsorption onto Activated Carbon
131
concentration in the bulk of the solution, CL*: solute
concentration in the solution at the interface.
2.3 Experiment
The acid violet 17 was obtained from a local
chemical supplier. The activated carbon, having
particle size <100 µm and bulk density of 150 - 440
kg/m3, was purchased from Merck Millipore. In all
batch adsorption experiments, activated carbon and
acid violet 17 were mixed in 250 ml beaker while
stirring continuously at a constant speed, in which the
volume of acid violet 17 solution was 100 ml.
Experimental variables include: initial concentration
of acid violet 17 (75 ppm, 125 ppm, 175 ppm, 225
ppm, 275 ppm) and mass of activated carbon used
(0.1 g; 0.1125 g; 0.125 g; 0.1375 g; 0.15 g). The
concentration of acid violet 17 in solution at certain
times and equilibrium was measured using a UV-Vis
spectrophotometer - Agilent 8453.
3 RESULTS AND DISCUSSION
3.1 Equilibrium Analysis
In this study, the equilibrium condition was assumed
to be achieved by allowing the batch adsorption
process to take place overnight. In this study, an
investigation of three equilibrium models was carried
out, i.e., Langmuir, Freundlich, and linear
equilibrium. The plot of equilibrium data obtained
from the experiment and plot of equilibrium models
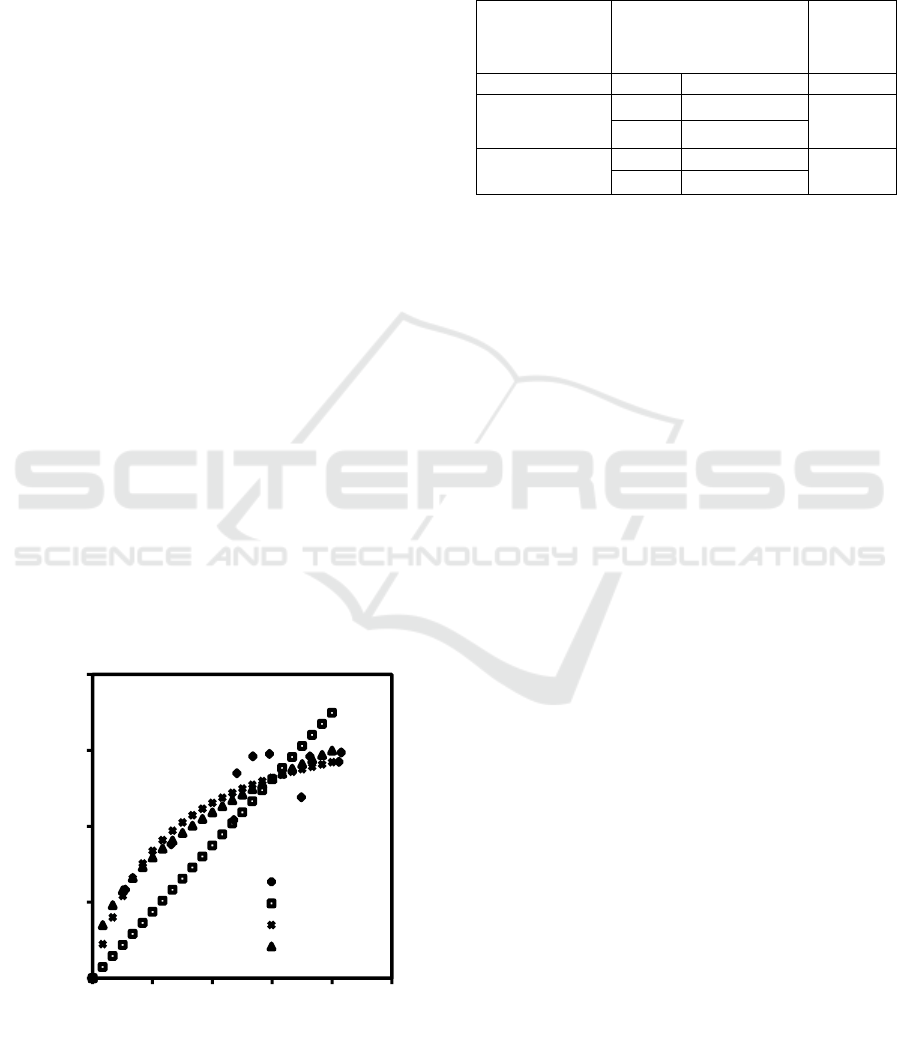
is presented in Figure 1.
Figure 1: Plot the experimental equilibrium data and the
equilibrium model.
The equilibrium parameters and the coefficient of
determination (R2) for each equilibrium model
observed for this system are presented in Table 1.
Table 1: The equilibrium parameters obtained for each
observed equilibrium model.
The observed
equilibrium
model
The equilibrium
parameters
R
2
Linear H 0.69 g/l 0.78
Freundlich
K 16.76
0.88
1
/
n
0.48
Langmuir
k 0,28 l/mg
0.93
C
μ
,max
185.19 mg/g
From the coefficient of determination, it can be
concluded that the Langmuir equilibrium model is the
most appropriate for this system. This result has
shown that the adsorption process takes place on a
homogeneous solid surface, and the adsorbed dyes
form a monolayer on the surface of activated carbon.
Furthermore, based on Langmuir's equilibrium
parameters obtained from this experiment, it can be
seen that the maximum adsorption capacity of the
activated carbon is 185.19 mg/g.
3.2 Kinetic Analysis
Kinetic models that are often used to observe
adsorption kinetic include the pseudo-first-order and
pseudo-second-order equations. The advantage of the
model is that the equation is simple, but both models
ignore the mass transfer or diffusion step that occurs
in adsorption and only taking into account the rate of
attachment of the solute to the surface of the
adsorbent (Yao & Chen, 2018).
In contrast, in this study the rate of attachment of
solutes to the surface of the adsorbent was considered
to be very fast so that the rate of the adsorption was
controlled by the rate of solute transfer across the
liquid film to the outer surface of the adsorbent and/or
the rate of solute diffusion within adsorbent particles.
The governing equations for the mass transfer in
the liquid film and the intraparticle diffusion are
partial differential equations, as expressed in the first
and second kinetic models. To obtain the mass
transfer coefficient of the solute in the liquid film and
the effective diffusivity of the solute within the
particles, the two models, are solved numerically
using the Matlab program.
0
50
100
150
200
0306090120150
Cμ,eq, mg/g
Ceq, ppm
Data
Linear
Langmuir
Freundlich
ICONIT 2019 - International Conference on Industrial Technology
132
3.2.1 The Solution of the First Kinetic
Model
The kinetic solution, which is expressed as changes in
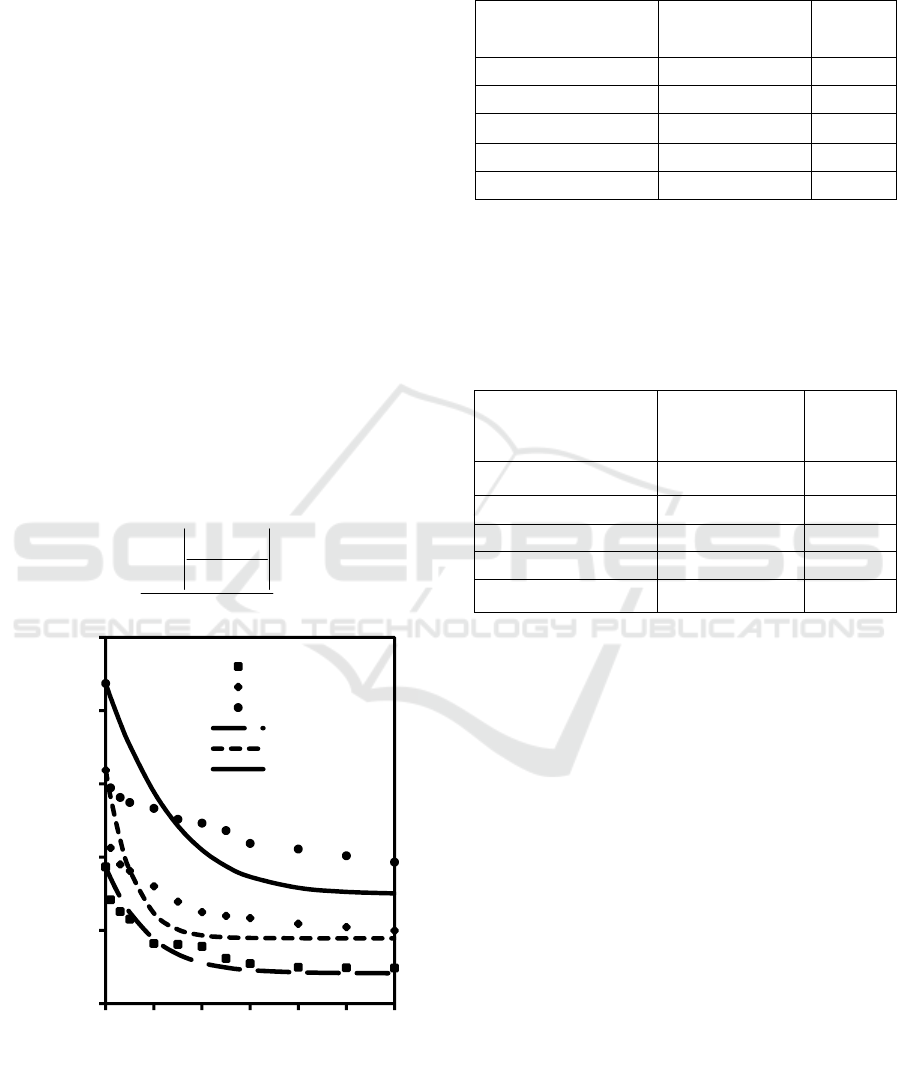
solute concentration in liquid bulk with respect to
time, for the first model, is presented in Figure 2. The
solution applied the Langmuir model for the
calculation of concentration in the interface.
From Figure 2, it can be seen that for lower
concentrations, the first model kinetic is better suited
to this adsorption system. It is shown that for lower
solute concentrations, the results obtained from the
model are closer to the data obtained from the
experiment.
Meanwhile, the mass transfer coefficient of liquid
films (kc), obtained from the application of the first
kinetic model at an experiment with an adsorbent
mass 0.1 gram, was presented in Table 2.
The AAD parameter is the absolute average
deviation used to determine the level of suitability of
the model to experimental data. The AAD parameter
is defined in Equation 9, where: Ce = dyes
concentration obtained from the experimental result,
Cm = dyes concentration obtained from the observed
kinetic model, N = the number of the experimental
data.
(9)
N
C
CC
AAD
N
i
e
me
Figure 2: Plot solute concentration in the liquid bulk versus
time at various initial solute concentrations. The results of
the first kinetic model were expressed as line, while the
experimental data were expressed as dot.
Table 2: Mass transfer coefficient at various solute initial
concentration applying the first kinetic model at an
experiment with an adsorbent mass 0.1 gram.
Meanwhile, the kc values at a various ratio of the
adsorbent mass to the solution volume are presented
in Table 3.
Table 3: Mass transfer coefficient at various ratio adsorbent
mass to solution volume applying the first kinetics model
when the initial solute concentration 275 ppm.
From the results obtained, it can be seen that the
change in solute concentration and the ratio of the
mass of adsorbent to the volume of solution does not
significantly influence the mass transfer coefficient.
The value of the mass transfer coefficient is in the
range of 8.38 x 10-5 - 1.97 x 10-4 cm/minute, with
the absolute average deviation in the range of 15.7%
- 25.1%.
3.2.2 The Solution of the Second Kinetic
Model
The value of the mass transfer coefficient and
effective diffusivity obtained by applying the second
kinetic model are presented in Table 4 for various
initial solute concentrations and Table 5 for the
various ratio of the mass of adsorbent to the volume
of solution.
Initial concentration
(ppm)
kc
(cm/minute)
AAD
(%)
75 1.24 x 10-4 25.1
125 1.97 x 10-4 19.7
175 1.37 x 10-4 17.9
225 8.66 x 10-5 16.5
275 8.55 x 10-5 19.2
Ratio the adsorbent
mass to the solution
volume (g/ml)
kc
(cm/minute)
AAD
(%)
0.001 8.56 x 10-5 15.7
0.001125 8.38 x 10-5 22.0
0.00125 1.05 x 10-4 23.2
0.001375 9.46 x 10-5 22.5
0.0015 8.78 x 10-5 22.6
0
40
80
120
160
200
0 102030405060
Solut concentration in liquid bulk (ppm)
Time(minutes)
data 75
data 125
data 175
75 ppm
125 ppm
175 ppm
Mass Transfer Modeling of Acid Violet 17 Adsorption onto Activated Carbon
133
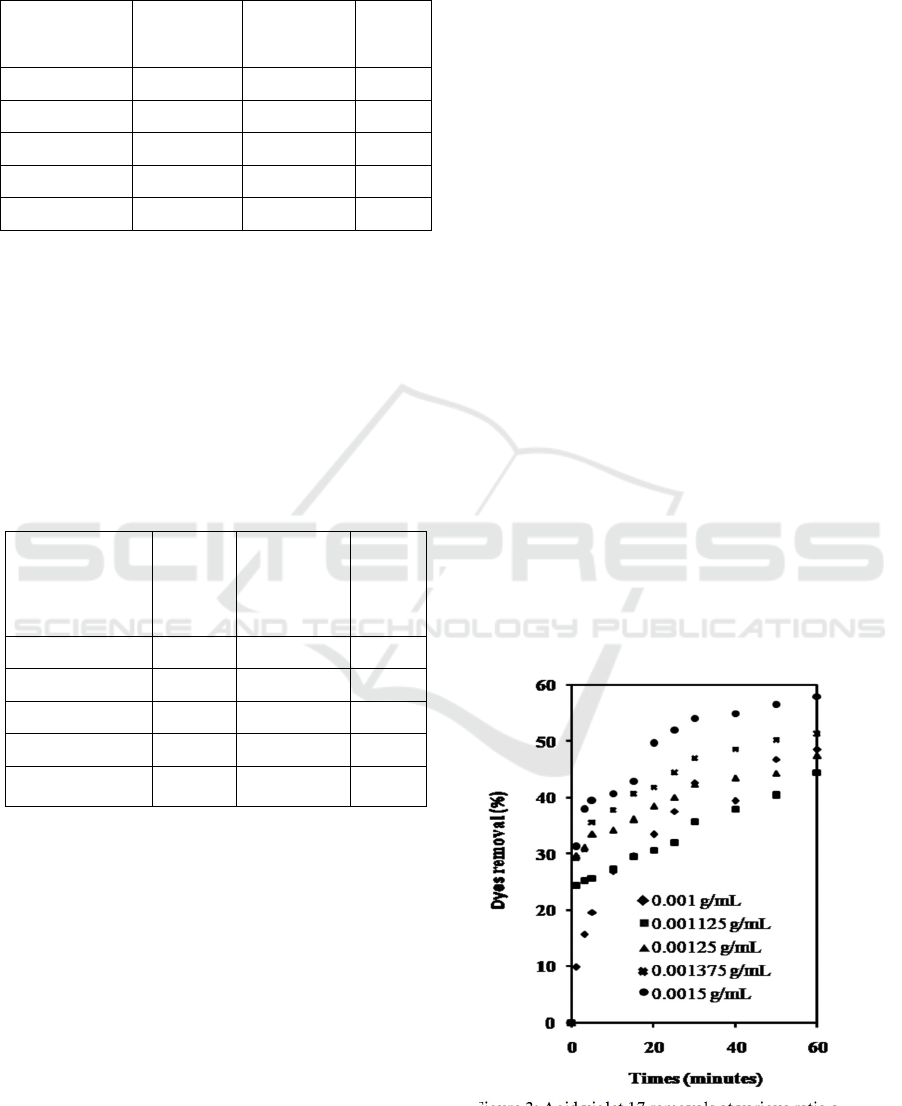
Table 4: The value of the mass transfer coefficient and
effective diffusivity obtained by applying the second
kinetic model for various initial solute concentrations.
Initial
concentration
(ppm)
kc
cm/minute)
De
(cm2/
minute)
AAD
(%)
75 0.28 2.13 x 10-5 7.6
125 0.25 2.13 x 10-5 13.5
175 0.19 1.06 x 10-5 14.4
225 0.15 7.38 x 10-6 7.9
275 0.10 5.52 x 10-6 9.8
By applying the second kinetic model, the mass
transfer coefficient value tends to decrease with
increasing solute concentration. It also found that the
ratio of the adsorbent mass to the solution volume
does not have much effect on the mass transfer
coefficient.
Table 5: The value of the mass transfer coefficient and
effective diffusivity obtained by applying the second
kinetic model for the various ratio of the mass of adsorbent
to the volume of the solution when the initial solute
concentration 275 ppm.
Ratio
the adsorbent
mass to the
solution volume
(g/ml)
kc
(cm/
minute)
De
(cm2/
minute)
AAD
(%)
0.001 0.13 6.54 x 10-6 4.1
0.001125 0.12 6.06 x 10-6 9.7
0.00125 0.11 8.22 x 10-6 12.6
0.001375 0.10 1.05 x 10-5 12.2
0.0015 0.10 1.36 x 10-5 11.8
The mass transfer coefficient obtained by
applying the second kinetic model is in the range of
0.10 – 0.28 cm/minute. This value is far greater than
the mass transfer coefficient obtained from the
application of the first kinetic model, which is in the
range of 8.38 x 10-5 - 1.97 x 10-4 cm/minute.
Meanwhile, the value of the effective diffusivity
tends to decrease from 2.13 x 10-5 cm2/minute to
5.52 x 10-6 cm2/minute when the solute
concentration increases from 75 ppm to 275 ppm and
it tends to increase from 6.54 x 10-6 cm2/minute to
1.36 x 10-5 cm2/minute when the ratio of the
adsorbent mass to the solution volume is increased
from 0.001 g/ml to 0.0015 g/ml.
When the second kinetic model was applied, the
absolute average deviation value is in the range of
4.1% -14.4%. It was much lower than its value when
the first kinetic model was applied, which was in the
range of 15.7% - 25.1%. This shows that, compared
to the first model, the second model is more suitable
for this case. Furthermore, these results also show that
in this case, the transfer of solutes into the particle is
controlled by the solute diffusion step within the
adsorbent particle. The determining factor for the
controlling stage is the pore structure of the particles.
The pore structure will determine the resistance of
solute diffusion within the particle.
3.3 Dyes Removal
The ability of adsorbent to absorb dyes is shown by
its removal value. Basically, the removal of the dye is
the mass of the dyes separated from the solution to the
initial mass of the dye.
The acid violet removal profile overtime at
various mass fractions of the adsorbent in the solution
when the initial solute concentration 275 ppm was
presented in Figure 3. The profile shows that up to 60
minutes, the percentage of dyes removal is still
increasing. This means that up to 60 minutes, the
adsorption process has not finished.
It can also be seen that the greater the ratio of the
mass of adsorbent to the volume of solution, the
greater the removal of the dyes. The highest removal
achieved was 58% when used 0.0015 g adsorbent per
ml solution.
Figure 3: Acid violet 17 removals at various ratio adsorbent
mass to solution volume, when the initial solute
concentration 275 ppm.
ICONIT 2019 - International Conference on Industrial Technology
134

4 CONCLUSIONS
Adsorption of acid violet 17 to activated carbon takes
place on a homogeneous solid surface form a single
layer at the surface. The maximum dyes removal
capacity is 185.19 mg/g. From the kinetic study, it
was known that the mechanism of acid violet 17
adsorptions on activated carbon is determined by the
diffusion stage in the particle. The percent dyes
removal was 58% achieved when 0.0015 g adsorbent
was used per ml solution.
ACKNOWLEDGMENTS
A deep gratitude is given to Stephanus Wibowo and
Andrew Riyadi S. for their full support during the
completion of this research works.
REFERENCES
Abd-Elhamid, A.I., Kamoun, E.A., El-Shanshory, A.A.,
Soliman, H.M.A. & Aly, H.F., 2019. Evaluation of
graphene oxide-activated carbon as effective composite
adsorbent toward the removal of cationic dyes:
Composite preparation, characterization and adsorption
parameters. Journal of Molecular Liquids 279, 530-539.
Belbel, B., Kharroubia, M., Janot, J., Abdessamad, M.,
Haouzi, A., Lefkaier, I.K. & Balme, S., 2018.
Preparation and characterization of homoionic
montmorillonite modified with ionic liquid:
Application in dye adsorption. Colloids and Surfaces A:
Physicochem. Eng. Aspects 558, 219-227.
Benabi, S., Streit, A.F.M., Benguerb, Y., Dotto, G.L., Erto,
A. & Ernst, B., 2019. Molecular modeling of anionic
and cationic dyes adsorption on sludge derived
activated carbon. Journal of Molecular Liquids 289, 1-
8.
Boudechiche, N., Fares, M., Ouyahia, S., Yazid, H., Trari,
M. & Sadaoui, Z., 2019. Comparative study on removal
of two basic dyes in aqueous medium by adsorption
using activated carbon from Ziziphus lotus stones.
Microchemical Journal 146, 1010-1018.
Daoud, M., Benturki, O., Girods, P., Donnot, A. & Fontana,
S., 2019. Adsorption ability of activated carbons from
Phoenix dactylifera rachis and Ziziphus jujube stones
for the removal of commercial dye and the treatment of
dyestuff wastewater. Microchemical Journal 148, 493-
502.
Dotto, G.L., Buriol, C. & Pinto, L.A.A. (2014) Diffusional
mass transfer model for the adsorption of food dyes on
chitosan films. Chemical Engineering Research and
Design 92, 2324-2332.
Ferreira, Guilherme M.D., Ferreira, Gabriel M.D.,
Hespanhol, M.C., Rezende, J., Pires, A.C. , Gurgel,
L.V.A & Mendes da Silva, L.H., 2017. Adsorption of
red azo dyes on multi-walled carbon nanotubes and
activated carbon: A thermodynamic study. Colloids
and Surfaces A: Physicochem. Eng. Aspects 529, 531-
540.
Gamoudi, S. & Srasra, E., 2019. Adsorption of organic
dyes by HDPyþ-modified clay: Effect of molecular
structure on the adsorption. Journal of Molecular
Structure 1193, 522-531.
Gao, Y., Deng, S., Jin, X., Cai, S., Zheng, S. & Zhang, W.,
2019. The construction of amorphous metal-organic
cage-based solid for rapid dye adsorption and time-
dependent dye separation from water. Chemical
Engineering Journal 357, 129-139.
Goswami, M. & Phukan, P., 2017. Enhanced adsorption of
cationic dyes using sulfonic acid modified activated
carbon. Journal of Environmental Chemical
Engineering 5 (4), 3508-3517.
Herrera-González, A.M., Caldera-Villalobos, M. & Peláez-
Cid, A., 2019. Adsorption of textile dyes using an
activated carbon and crosslinked polyvinyl phosphonic
acid composite. Journal of Environmental Management
234, 237-244.
Jain, S.N. & Gogate, P.R., 2017. Adsorptive removal of
acid violet 17 dye from wastewater using biosorbent
obtained from NaOH and H2SO4 activation of fallen
leaves of Ficus racemosa. Journal of Molecular Liquids
243, 132–143.
Kausar, A., Iqbal, M., Javed, A., Aftab, K., Nazli, Z., Bhatti,
H.N. & Nouren, S. (2018) Dyes adsorption using clay
and modified clay: A review. Journal of Molecular
Liquids 256, 395-407.
Khorasani, A.C. & Shojaosadati, S.A., 2019. Magnetic
pectin-Chlorella vulgaris biosorbent for the adsorption
of dyes. Journal of Environmental Chemical
Engineering 7 (3), 1-8.
Liu, K., Deng, L., Li, H., Bao, Y., Xiao, Z., Li, B., Zhou,
Q., Geng, Y. & Wang, L., 2019. Two isostructural
Co/Ni fluorine-containing metal-organic frameworks
for dye adsorption and supercapacitor. Journal of Solid
State Chemistry 275, 1-7.
Li, W., Mu, B. & Yang, Y. (2019) Feasibility of industrial-
scale treatment of dye wastewater via bioadsorption
technology. Bioresource Technology 277, 157-170.
Magdy, Y.H. & Altaher, H., 2018. Kinetic analysis of the
adsorption of dyes from high strength wastewater on
cement kiln dust. Journal of Environmental Chemical
Engineering 6 (1), 834-841.
Ocampo-Perez, R., Leyva-Ramos, R., Alonso-Davila, P.,
Rivera-Utrilla, J. & Sanchez-Polo, M., 2010. Modeling
adsorption rate of pyridine onto granular activated
carbon. Chemical Engineering Journal 165, 133-141.
Ocampo-Perez, R., Leyva-Ramos, R., Mendoza-Barron, J.
& Guerrero-Coronado, R.M., 2011. Adsorption rate of
phenol from aqueous solution onto organobentonite:
Surface diffusion and kinetic models. Journal of
Colloid and Interface Science 364, 195-204.
Ocampo-Pérez, R., Rivera-Utrilla, J., Gómez-Pacheco, C.,
Sánchez-Polo, M. & López-Peñalver, J.J., 2012.
Kinetic study of tetracycline adsorption on sludge-
Mass Transfer Modeling of Acid Violet 17 Adsorption onto Activated Carbon
135

derived adsorbents in aqueous phase. Chemical
Engineering Journal 213, 88-96.
Rajabi, M., Mahanpoor, K. & Moradi, O., 2019.
Preparation of PMMA/GO and PMMA/GO-Fe3O4
nanocomposites for malachite green dye adsorption:
Kinetic and thermodynamic studies. Composites Part
B: Engineering 167, 544-555.
Saleem, M., Pirzada, T. & Qadeer, R., 2007. Sorption of
acid violet 17 and direct red 80 dyes on cotton fiber
from aqueous solutions. Colloids and Surfaces A:
Physicochem. Eng. Aspects 292, 246–250.
Thinakaran, N., Baskaralingam, P., Pulikesi, M.,
Panneerselvam, P. & Sivanesan, S., 2008. Removal of
Acid Violet 17 from aqueous solutions by adsorption
onto activated carbon prepared from sunflower seed
hull. Journal of Hazardous Materials 151, 316–322.
Vahdati-Khajeh, S., Zirak, M., Tejrag, R.Z., Fathi, A.,
Lamei, K. & Eftekhari-Sis, B., 2019. Biocompatible
magnetic N-rich activated carbon from egg white
biomass and sucrose: Preparation, characterization and
investigation of dye adsorption capacity from aqueous
solution. Surfaces and Interfaces 15, 157-165.
Yao, C. & Chen, T., 2018. A weighted average kinetic
equation and its application in estimating mass transfer
coefficients in liquid phase adsorption. Biophysical
Chemistry 241, 50-54.
Yu, Y., Qiao, N., Wang, D., Zhu, Q, Fu, F., Cao, R. Wang,
R., Liu, W. & Xu, B., 2019. Fluffy honeycomb-like
activated carbon from popcorn with high surface area
and well-developed porosity for ultra-high efficiency
adsorption of organic dyes. Bioresource Technology
285, 2-10.
ICONIT 2019 - International Conference on Industrial Technology
136
