
Biodiversity of Endophytic Fungi in Sembilang National Park of
South Sumatera
Rifka Rimbi Anggraini
1*
, Uun Yanuhar
2
, Yenny Risjani
2
1
Master Program in Faculty of Fisheries and Marine Science, Brawijaya University, Jalan Veteran Kota Malang 65145,
Indonesia
2
Departement of Aquatic Resource Management, Faculty of Fisheries and Marine Science, Brawijaya University, Jalan
Veteran Kota Malang 65145, Indonesia
*corresponding author: Rifka Rimbi Anggraini
Keyword: Bruguiera gymnorrhiza, Endophytic fungi, Sembilang National Park
Abstract: Endophytic fungi originating from areas affected by tidal water are microbes that are rich in natural bioactive
products and secondary metabolites. The purpose of this study was to determine endophytic fungi that live in
symbiosis with mangrove plants from Bruguiera gymnorrhiza species taken from the Sembilang National
Park, South Sumatra. The research method in isolating symbiont mushrooms was carried out using the Direct
Planting method with PDA (Potato Dextrose Agar) media. The results in this study indicate that there are
three types of endophytic fungi, namely Aspergillus flavus, Penicillium sp., and Aspergillus niger.
1 INTRODUCTION
The potential possessed by the diversity of natural
resources, especially plants, still needs to be studied.
According to Prihatiningtias (2005), sources of
bioactive compounds are obtained from plants,
animals, microbes and marine organisms which are
continuously being explored as more and more new
diseases emerge. These endophytic microbes were
first discovered by Darnel et al on 1904 and from then
on, the definition of endophytic microbes was agreed
as microorganisms that live in plant tissue systems
and symbiotic mutualism (Stone et al., 2000).
Endophytic fungi are one of the endophytic
microbial organisms (Strobel, 2003). In-plant tissues
that have endophytic fungi can produce compounds
that have the same properties as the host plant,
although the types of compounds are different. The
activity of compounds produced by endophytic fungi
is usually greater than that of the host compound
(Strobel et al., 2004).
One of the plants that contain a lot of bioactive
compounds produced by endophytic fungi in
mangrove plants. According to several researchers in
Noor et al. (2012) mangroves are plants that live
between sea and land, in the form of shrubs and trees
and at high tide, the roots of these mangroves will be
flooded by water and the receding time of the roots
will be seen. Bruguiera gymnorrhiza is a species of
mangrove that grows on muddy soils, is flooded
during high tide and does not like hard substrates such
as sand.
So far, many researchers have succeeded in
isolating endophytic fungi and secondary metabolite
compounds from various types of plants. However,
researchers who isolate endophytic fungi from B.
gymnorrhiza mangroves and information on
endophytic fungi in mangroves as producers of
natural ingredients are still limited in Indonesia,
especially in the Mangrove Ecosystem in South
Sumatra. Limited information, the authors have
conducted research on the isolation of endophytic
fungi in mangrove B. gymnorrhiza plants taken from
the mangrove area of the Sembilang National Park in
South Sumatera.
2 MATERIALS AND METHODS
The research sample was taken using a purposive
sampling method which is located in Sembilang
National Park, South Sumatera (Fig. 1).
Anggraini, R., Yanuhar, U. and Risjani, Y.
Biodiversity of Endophytic Fungi in Sembilang National Park of South Sumatera.
DOI: 10.5220/0009588001050111
In Proceedings of the 6th International Conference on Advanced Molecular Bioscience and Biomedical Engineering (ICAMBBE 2019) - Bio-Prospecting Natural Biological Compounds for
Seeds Vaccine and Drug Discovery, pages 105-111
ISBN: 978-989-758-483-1
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
105

Figure 1. Research Location
The tools used in this research are aluminum
foil, autoclave, identification book, bunsen, petri dish,
cover glass, cutter, erlenmeyer, freezer, measuring
cup, hot plate, incubator, inoculating loop, cotton,
filter paper, laminar air flaw, masks, microscopes,
analytical balances, glass objects, tweezers, and
plastic wrap. The materials used in this study were
apart, sterile seawater, distilled water, 70% alcohol,
dextrose, 75% ethanol, seawater, chloramphenicol,
lactofenol blue cotton, potato dextrose broth (PDB),
potato dextrose agar (PDA), Bruguiera gymnorrhiza
and spiritus.
2.1 Collection and Preparation of
Bruguiera Gymnorrhiza Samples
B. gymnorrhiza mangrove samples were randomly
selected from one of the tree representatives in the
mangrove zoning of the Sembilang National Park
area, South Sumatra. Mangrove samples taken are the
roots, stems and leaves as much as ± 500 gram each
part (Fig. 2). The sample taken is put into a sterile
plastic sample so that the sample is not contaminated,
then put in a cool box. In handling in the laboratory,
samples that have been taken are washed using sterile
seawater 3 times to remove impurities. Furthermore,
soaked using 70% alcohol for 1-2 minutes to kill the
epiphytic fungus that sticks to the surface. After that,
the samples were rinsed again using sterile seawater
(Kjer et al., 2010).
(a)
(b)
(c)
Figure 2: (a) Leave, (b) Root and (c) Stems of Bruguiera
gymnorrhiza (Personal Docummentation)
2.2 The Manufacture of Media Growth
Endophytic Fungi
In this research, there are two media used, namely
Potato Dextrose Broth (PDB) as liquid media and
Potato Dextrose Agar (PDA) as solid media. The way
of making these media is as follows
:
2.2.1 Potato Dextrose Broth (PDB)
12 gram PDB dissolved with 500 ml of seawater in an
erlenmeyer tube. The erlenmeyer tube is closed using
a cotton swab that is coated with aluminium foil.
Seawater and media are homogeneous using a hot
plate. The media is waited until completely
homogeneous. Then the media is sterilized by
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
106

autoclave for 15 minutes with a temperature of 121
o
C
and a pressure of 1 atm (Ariyono et al., 2014).
2.2.2 Potato Dextrose Agar (PDA)
PDA media as much as 19.5 gram in 500 ml of
seawater was dissolved in an erlenmeyer tube. The
erlenmeyer tube is closed using a cotton swab that is
coated with aluminium foil. Seawater and media are
homogeneous using a hot plate. The media is waited
until completely homogeneous. Then the media is
sterilized by autoclave for 15 minutes with a
temperature of 121
o
C and a pressure of 1 atm. Then
chill the media a few moments then put
Chloramfenicol as much as 0.1 gram. Modifications
from Ariyono et al. (2014).
2.3 Growth of Endophytic Fungi
Isolates
Samples (roots, leaves and stems) were cut to size ±
1x1 cm. The sample was put into the GDP medium
with a ratio of 1: 9 (g/v) where 10 gram of sample was
added 90 mL of PDB media. Samples are stirred
using a shaker for 4-7 days until the colour of the
water turns brownish turbid at a speed of 150 rpm at
room temperature (Kjer et al., 2010).
Samples on PDB media are put into test tubes with
the principle of multilevel dilution. The last three
dilutions (10
-4
,10
-5
and 10
-6
) were taken for planting
by the pouring method on 1 mL petri dishes. PDA
media that have been made are taken, poured into a
petri dish while homogenized until the media
becomes solid and incubated for 7 days at 25
o
C
(Benson, 2002).
2.4 Purification of Endophytic Fungi in
Potato Dextrose Agar (PDA) Media
Fungal colonies that have grown on PDA media were
previously regrown on sterile PDA media based on
morphological differences from each growing colony
to obtain endophytic fungal colonies according to
their respective morphology. Fungal colonies on
PDA media were taken using a sterile round
inoculating loop then etched on aseptic sterile PDA
media in laminar airflow. If one mushroom colony is
still mixed with other colonies, then it is refined
repeatedly until a pure mushroom colony is obtained
(Ariyono et al., 2014).
2.5 Characterization of Endophytic
Fungi
Characterization was carried out on each fungal
colony macroscopically and microscopically. The
results of observations are used as ingredients for
identification of endophytic fungi. Gandjar (1999)
mentions macroscopic observations include the
colour and surface of the colony, radial lines from the
centre of the colony towards the edge of the colony,
and concentric circles in concentric or non-concentric
Petri dishes and colony growth (cm/day).
Microscopic observations include hyphae bulkhead,
hyphae growth, hyphae colour, presence or absence
of conidia and conidia form. Microscopic
observations were made on the last day observations
(5-7 days) using a microscope.
This microscope observation was carried out
using the slide culture method. A sterile petri dish is
provided, a buffer ring is placed inside and 5 mL of
distilled water is added to maintain moisture. The top
of the ring is placed with glass preparations/object
glass and sterile PDA media pieces on it. Fungi
culture is taken and applied to the entire surface and
closed using a glass cover. Fungi cultures were
incubated for 5-7 days at 25
o
C. Cultures that have
grown on the cover glass are placed at the top of the
glass preparation which is dripped with lactofenol
blue cotton to increase the transparent effect on the
fungi to be more easily observed under a microscope
at magnifications of 10X and 40X (BKIPM, 2014).
2.6 Identification of Endophytic Fungi
Observations obtained from the characterization of
fungi will be used observation results obtained from
the characterization will be used for the identification
stage based on the guide book identification
Introduction to Food-Borne Fungi (Samson et al.,
1995), Introduction of General Tropical Molds
(Gandjar et al., 1999) and Identifying Filamentous
Fungi (St-Germain and Summerbell, 1996).
3 RESULT
3.1 Endophytic Fungi Pure Colony
In the growth of fungi in PDA media for 7 days at a
temperature of 25ºC found seven types of pure isolates
in mangrove B. gymorrhiza. Of the six types, there are
three types of isolates that differ in shape, colour and
texture. There are several differences in visually
purified endophytic fungi. The macroscopic
Biodiversity of Endophytic Fungi in Sembilang National Park of South Sumatera
107
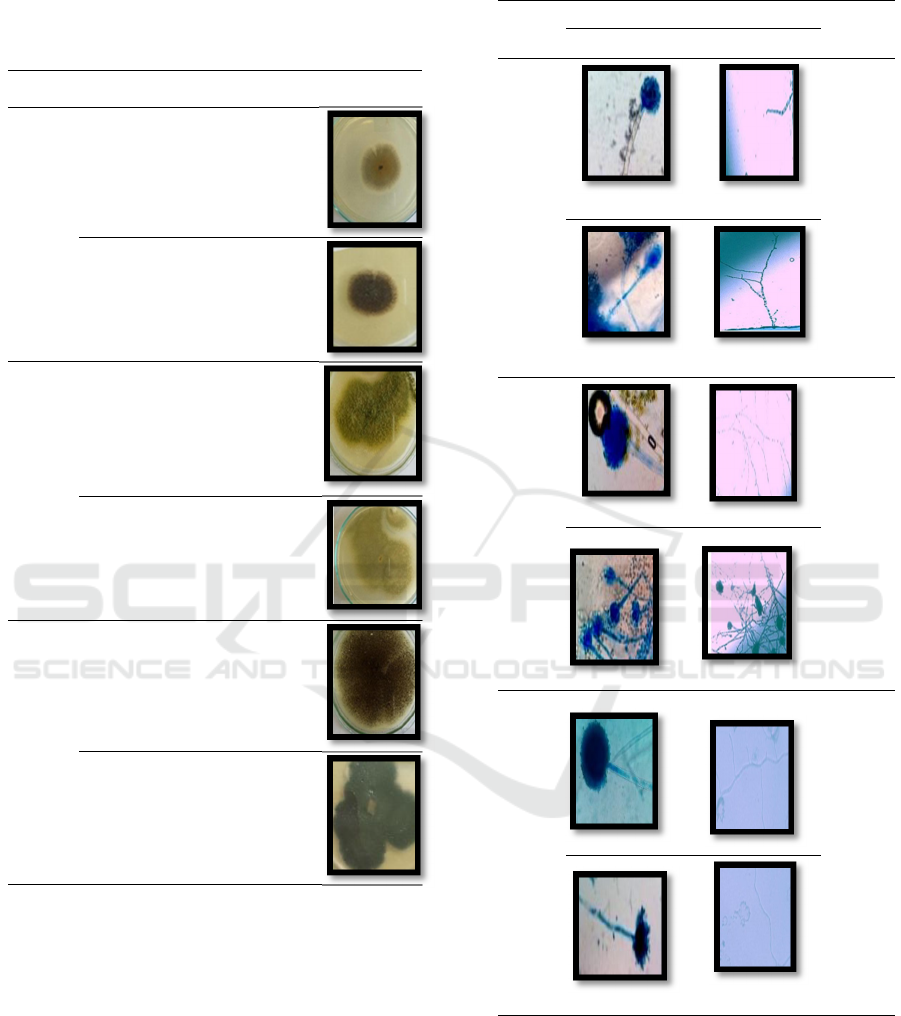
observation of endophytic fungi can be seen in Table
1.
Table 1. Macroscopic Characteristics of Endophytic Fungi
Organ Shape Colour Texture Figure
Stem
Round Brownish Smooth
Powder
Round Black As if
cotton
Root
Round Green Velvety
Round White Smooth
Powder
Leaf
Round White Smooth
Powder
Round Black Velvety
Fungi that have been made macroscopic
observations with visual observations, then the
microscopic observation stage is carried out to make
it easier to identify the results that have been
obtained. Microscopic characterization is a
continuation of the stage of identifying fungi. This
observation was carried out by observing hyphae,
spores, and conidia formed under a microscope lens
with a magnification of 400X. Macroscopic
observations can be seen in Table 2.
Table 2. Microscopic Characteristics of Endophytic Fungi
Organ Figure Expla-
nation
Sporae Hyphae
Stem
The
hyphae
form has
a divider
and is
branched
on
hyaline
Conidia
are
round
and
black
Root
The
hyphae
form has
a divider
and
is
branched
on
hyaline
Conidia
are round
and
black
Leaf
The
hyphae
form has
a divider
and is
branched
on
hyaline
Conidia
are round
and
black
4 DISCUSSION
In general, the fungi has been found to grow clearly.
Pure colonies were obtained based on differences in
the visual appearance of each isolate. The fungi
obtained at the stems has a brownish to black colour,
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
108

smooth texture like cotton. The diameter size of fungi
colonies taken from stems tends to be smaller because
the growth rate is relatively slow. The fungi obtained
at the root looks even more different from other types
of fungi because of the difference in colour. This
fungi has a green colour with a larger colony diameter
than fungi obtained from the leaves. The speed of
growth of this fungi is also relatively faster than the
fungi from the isolation of the leaves.
The fungi obtained from the leaves has a black
spore colour. The size of the diameter of this fungi
colony is the biggest compared to the fungi isolated
from the stem and roots. The speed of growth of this
fungi is much faster compared to other fungi.
Therefore, the type of fungi obtained from these
leaves grows to meet the entire surface of the petri
dish.
The purification stage of the leaf part is more
abundant with endophytic fungi compared to roots
and stems. This is consistent with what was stated by
Noverita et al. (2009); Sinaga et al. (2009) where
more endophytic fungi isolates were obtained from
the leaves. This phenomenon is suspected because the
nutrients present in the leaves are more supportive of
the growth of endophytic fungi. Endophytic fungi
isolated from one host plant contain different types of
isolates, even from one living tissue obtained from a
plant can be isolated more than 1 type of endophytic
fungi. This is an adaptation mechanism of endophytic
fungi to the microecology and specific physiological
conditions of each host plant.
Macroscopically, the fungi of Aspergillus niger is
found in the leaves. This fungi is black and has white
hyphae. Based on Summerbell (1996) that the
Aspergillus niger fungi grow rapidly until its diameter
fills the entire surface of the PDA media. The
Aspergillus niger fungi itself is black and fills the
entire surface of the media. This is also supported by
microscopic results in which the Aspergillus niger
fungi has the characteristics of large enough black
spores covering all spore bubbles where the conia
spreads tightly and has clear conidiospores. These
characteristics are also found in microscopic images
of the fungi of Aspergillus niger based on references
from the identification book Summerbell (1996).
The endophytic fungi of Aspergillus niger is a
fungus that is isolated from every organ of the B.
gymnorrhiza mangrove plant. The characteristics of
Aspergillus niger are having a large black conidia
head and a round shape. The hyphae were insulated
and branched mycelium. This fungi also have vesicles
(bubbles) at the ends of the conidiophores and
becomes a place of conidia to grow. The conidia
shaped chain and black. This fungi grows well at
room temperature (Wuryanti, 2008).
The fungi of Aspergillus niger is a type that
produces quite a lot of compounds and enzymes.
According to Handajani and Purwoko (2008), the
fungi of Aspergillus niger can produce ochratoxin
compounds and can produce lipase enzymes. Lipase
enzyme is an enzyme that plays an important role in
the world of modern biotechnology because it has
high activity in hydrolysis and synthesis and chemical
reactions.
Aspergillus niger fungi which belong to the group
of phosphate solvent fungi. Phosphate solvent fungi
are able to be used as biofertilizer which is the result
of biotechnology engineering in the field of soil
science. Aspergillus niger has the ability to dissolve
phosphate compounds that are difficult to dissolve
into a form available to plants by producing organic
acids so that availability becomes faster (Artha et al.
2013).
In addition to secondary metabolites produced by
Aspergillus niger, this fungi is also able to produce
cellulase enzymes (Sa'adah et al. 2010), proteases
(Ramdhani et al. 2015), and chitinase (Purkan et al.
2016). Protease enzymes produced by A. niger fungi
are able to be grouped in alkaline proteases which are
one of the groups of hydrolytic enzymes that is able
to catalyze the hydrolysis process or the proteins
damage into their constituent amino acids (Ramdhani
et al. 2015). The forms of commercial products in the
application of alkaline proteases in the industrial
sector include the detergent industry, the food
industry, the pharmaceutical industry, milk, skin, and
meat processing (Ramdhani et al. 2015).
Described in the book Gandjar et al. (1999)
Aspergillus flavus is a fungi commonly found in nuts
(especially peanuts), spices, oilseeds, cereals, and
sometimes in dried fruit. The fungi of Aspergillus
flavus is a fungi that is green and shaped like soft hair
because it has fairly long hyphae. This fungi has a
fairly large size because it almost fills the entire
surface of the PDA media. Microscopic observations
show that these fungi have quite large spores, with
conidial heads scattered throughout the bubble
surface and has rough conidiospores walls
(Summerbell, 1996).
A. flavus fungi is also a fungus that can produce
aflatoxin compounds. The main aflatoxin compounds
are produced by the fungi of A. flavus, which is one
of the causes of cancer in humans (Handajani and
Setyaningsih, 2006). In addition to aflatoxin
compounds, Setiarto (2011) also suggested that the
fungi of A. flavus can produce ochratoxin and
zearalenone compounds. In extreme conditions, this
Biodiversity of Endophytic Fungi in Sembilang National Park of South Sumatera
109

type of fungi can infect grains directly which can later
cause aflatoxin accumulation. This can cause health
problems in animals and even humans due to
contamination of feed ingredients by aflatoxins. A.
flavus fungi can also be used as antibacterial
metabolites. A. flavus can inhibit the growth of
Echercia coli which is a bacterium that causes
diarrhoea.
Based on the results of Hidayati's research (2010)
the selection of six endophytic fungi isolates
produced antibacterial metabolites using the Kirby-
Bauer test method where the results were all isolates
could form inhibitory zones against the test bacteria.
A. flavus fungi can inhibit the growth of E. coli
bacteria by 9.33 mm. Research Raharjo et al. (2007)
said that this fungus is able to dissolve phosphate
which cannot be dissolved so that plants can be used
in growth.
Gandjar et al. (1999) describe the fungi of
Penicillium sp. has a surface with a velvety texture
although sometimes like cotton. The colours in the
colonies are sometimes yellow to brownish, greyish-
green to yellowish-green and greyish green.
Conidiophores in fungi arise from the substrate and
generally has many branches and smooth-walled.
Habitat from this fungi is very common in various
food products, as well as food items that are low in
the water. Penicillium sp. used in industry to produce
antibiotics (Crystovel, 2017).
These fungi are known as fungi that produce
antibiotic metabolites. Amaria et al. (2013) said that
Penicillium, Trichoderma and Aspergillus are fungi
that can release antibiotic-like substances that can
inhibit the growth of pathogens so that these fungi are
antagonistic fungi that can be used as biopesticide and
biofertilizer fungi. Subowo (2015) explains that the
fungi of Penicillium sp. able to decompose cellulose
and lignin compounds into simple carbon compounds
needed by soil microbes as an energy source so that
this fungi is very good for soil fertility.
5 CONCLUSION
The results of isolation and identification of
endophytic fungi from Bruguiera gymnorrhiza
mangroves taken from the Sembilang National Park,
South Sumatera are known that there are two types of
fungi from the Aspergillus genus namely Aspergillus
niger and Aspergillus flavus, and one of the
Penicillium genera namely Penicillium sp.
REFERENCES
Amaria, W., Taufiq, E., Harni, R., 2013. Seleksi dan
identifikasi jamur antagonis sebagai agens hayati jamur
akar putih Rigidoporus microporus pada tanaman karet.
Jurnal Tanaman Industri dan Penyegar. 4 (1): 55 – 64.
Ariyono, R.Q., Djauhari, S., Sulistyowati, L., 2014.
Keanekaragaman jamur endofit daun kangkung darat
(Ipomoea reptans Poir.) pada lahan pertanian organik
dan konvensional. Jurnal Hama dan Penyakit
Tumbuhan. 2(1): 19-28.
Artha, P.J., Guchi, H., Marbun, P., 2013. Efektivitas
Aspergillus niger dan Penicillium sp. dalam
meningkatkan ketersediaan fosfat dan pertumbuhan
tanaman jagung pada tanah andiso. Jurnal
Agroekoteknologi Universitas Sumatera Utara. 1 (4):
1277 – 1287.
Benson, H.J., 2002. Microbiological Applications a
Laboratory Manual in General Microbiology. Boston:
McGraw Hill.
BKIPM. 2014. Instruksi Kerja Teknis Jamur. Palembang:
Balai Karantina Ikan Pengendalian Mutu dan
Keamanan Hasil Perikanan Kelas II Palembang.
Crystovel, J., 2017. Mikologi tanaman: Penicillium sp.,
Paecilomyces sp. dan Aspergillus sp. Sumedang:
Universitas Padjadjaran.
Gandjar, I., Samson, R.A., Twell-Vermeulen, Kvd., Oetari,
A., Santoso, I., 1999. Pengenalan Kapang Tropik
Umum. Jakarta: Yayasan Obor Indonesia.
Handajani, N.S., Purwoko, T., 2008. Aktivitas ekstrak
rimpang lengkuas (Alpinia galangal) terhadap
pertumbuhan jamur Aspergillus spp. penghasil
aflatoksin dan Fusarium moniliforme. Biodiversitas.
9(3): 161-164.
Handajani, N.S., Setyaningsih, R., 2006. Identifikasi jamur
dan deteksi Aflatoksin B1 terhadap petis udang
komersial. Biodiversitas. 7(3): 212-215.
Hidayati, U., 2014. Potensi bakteri Endofit Asal Pohon
Karet sebagai Pemacu Pertumbuhan Bibit Batang
Bawah Tanaman Karet (Hevea brasiliensis Müll. Arg.)
[Thesis]. Bogor: Sekolah Pascasarjana, Institut
Pertanian Bogor.
Kjer, J., Debbab, A., Aly, A.H., Proksch, P., 2010. Methods
for isolation of marine-derived endophytic fungi and
their bioactive secondary products. Nature Protocols.
5(3): 479-490.
Noor, Y.R., Khazali, M., Inn, S., 2012. Panduan
Pengenalan Mangrove di Indonesia: PKA/WI-IP
(Wetlands International-Indonesia Programme).
Noverita, F.D., Sinaga, E., Nasional, F.B.U., Manila, J.S.,
Pejaten, P.M., Selatan, J., 2009. Isolasi dan uji aktivitas
antibakteri jamur endofit dari daun dan rimpang
Zingiber ottensii Val. Jurnal Farmasi Indonesia. 4:
171-176.
Prihatiningtias, W., 2005. Senyawa bioaktif fungi endofit
akar kuning (Fibraurea Hloroleucac Miers) sebagai
senyawa antimikroba. [Thesis]. Yogyakarta:
Pascasarjana Universitas Gajah Mada.
Purkan, P., Baktir, A., Sayyidah, A.R., 2016. Produksi
Enzim Kitinase dari Aspergillus niger menggunakan
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
110

Limbah Cangkang Rajungan sebagai Induser. Journal
Kimia Riset, 1 (1): 34-41.
Raharjo, B., Suprihadi, A., Agustina, D., 2007. Pelarutan
fosfat anorganik oleh kultur campur jamur pelarut
fosfat secara in vitro. Jurnal Sains dan Matematika. 15
(2): 45 – 54.
Ramadhani, P., Rukmi, M.I., 2015. Produksi Enzim
Protease Dari A. niger PAM18A dengan Variasi pH dan
Waktu Inkubasi. Jurnal Biologi. 4 (2): 25 – 34.
Sa’adah, Z., Ika, S., 2010. Produksi Enzim Selulase oleh
Aspergillus niger Menggunakan Substrat Jerami
dengan Sistem Fermentasi Padat. Teknik Kimia. 1 (2):
1 – 10.
Samson, R.A., Hoekstra, E.S., Frisvad, J.C., Filtenborg, O.,
1995. Introduction To Food - Borne Fungi.
Netherlands: Centralbureau Voor Schimmelcutures.
Setiarto, R.H.B., 2011. Comparative Study Toxicity LC50
Aflatoxin Ochratoxin, Zearalenon in Peanut (Arachis
Hypogaea L). Widyariset. 14(3): 535-540.
Sinaga, E., Noverita, Fitria, D., 2009. Daya antibakteri
jamur endofit yang diisolasi dari daun dan rimpang
lengkuas (Alpinia galangal Sw.). Jurnal Farmasi
Indonesia. 4: 161-162.
St-Germain, G., Summerbell, R., 1996. Identifying
Filamentous Fungi: A Clinical Laboratory Handbook.
Star Publishing Company, Singapore.
Stone, J.K., Bacon, C.W., White, J., 2000. An overview of
endophytic microbes: endophytism defined.
Strobel, G.A., 2003. Endophytes as sources of bioactive
products Microbes and Infection. 5: 535-544.
Strobel, G., Daisy, B., Castillo, U., Harper, J., 2004. Natural
products from endophytic microorganisms. Journal of
Natural Products. 67(2): 257-268.
Subowo, Y., 2015. Pengujian aktifitas jamur Penicillium sp.
R7, 5 dan Aspergillus niger NK pada media tumbuh
untuk mendukung pertumbuhan tanaman padi di lahan
salin. Jurnal Pros Sem Nas Masy Biodiv Indos. 1 (5):
1136 – 1141.
Summerbell, R., 1996. Identifying filamentous fungi: a
clinical laboratory handbook: Star Publishing
Company.
Wuryanti. 2008. Pengaruh penambahan biotin pada media
pertumbuhan terhadap produksi sel Aspergillus niger.
Jurnal Bioma. 10(2): 46-50.
Biodiversity of Endophytic Fungi in Sembilang National Park of South Sumatera
111
