
Lactonization Castor Oil (Ricinus Communis) using Lipase B from
Candida Antarctica Recombined Aspergillus oryzae as Bioflavor
Galuh Alya Stywarni
1
, Elvina Dhiaul Iftitah
1
and Arie Srihardyastutie
1
1
Department of Chemistry, Faculty of Mathematics and Science, Brawijaya
University, Malang, Indonesia
Keywords: Castor Oil (Ricinus communis), Lactonization, Lipase, Bioflavor.
Abstract: Lactone is a widely flavor that is used in food production. Lactonization using microbial or enzyme has natural
labelled products, has a higher economic value than artificial products and is safe for the environment.
Lactonization of castor oil (Ricinus communis) using lipase B from Candida antarctica recombined
Aspergillus oryzae (T = room, 40ºC) for 24, 48 and 72 h were investigated. The lactonization reaction was
carried out using a magnetic hotplate stirrer with the reaction system consisting of castor oil, n-hexane solvent,
Na2CO3 solution, and lipase biocatalyst. Lactonization castor oil products were analysed using GC-MS. At
T = room, the major products were ester: methyl ricinoleate, 53.64% (t = 24 h) and other products were fatty
acids and lactone. Lactone: γ-dodecalactone, 1.75% (t = 48 h) was a minor product. Whereas at T = 40ºC,
only produced ester, the major product was methyl ricinoleate, 81.33% (t = 72 h).
1 INTRODUCTION
One of the potential sources of natural-based raw
materials that are widely used in industry is castor oil.
Castor oil consists of thick yellow liquid, has a
characteristic odour with a molecular weight of
933.45 g/mol, a density of 0.95 g / cm3 and a boiling
point of 313°C (Moradi et al., 2013). The content of
castor oil consists of ricinoleic acid, linoleic acid,
oleic acid, stearic acid, palmitic acid,
dihydroxystearic acid, linoleic acid, and eicosanoic
acid (Farbood and Willis, 1985). The main
component of castor oil is ricinolein, a glyceride
from ricinoleic acid. Ricinoleic acid has three
functional groups namely ester linkage, double
bonds and hydroxyl groups which are used as
sources of renewable raw materials in chemical
reactions, modification, and transformation into
useful products (Wache et al., 2001). In the food
industry, castor oil potential produces bioflavor.
Various lipase-producing microbes have been
reported as catalyse bioflavor (γ-decalactone) using
castor oil substrate or ricinoleic acid (12-hydroxy-9-
octadecenoic acid).
γ-Decalactone as a flavouring agent has fruit,
creamy, peach, apricot, and fatty taste. In enzymatic
biotransformation, the substrate is degraded through
α-oxidation to produce 4-hydroxidecanoic acid, then
cyclization to
γ-decalactone (Gutman et al., 1989)
.
Based on research by Gotz et al. (2013), immobilized
B lipase from Candida antarctica able to catalyse the
formation of (S) -γ-valerolactone from a substrate
(S) -ethyl-4- hydroxy pentanoate with a yield of
90% (Antczak et al., 1991). According to Gutman
et al. (1989), the lactonization reaction rate affected
by the hydrophobicity of the solvent, n-hexane
solvent is two times faster than ether and four times
faster than chloroform (Khan and Rathod, 2018).
2 MATERIALS AND METHODS
2.1 Chemicals and Enzymes
Candida antarctica lipase B (recombinant from
Aspergillus oryzae) (1800 U/gram), n- hexane, and
sodium carbonate were obtained from Sigma-
Aldrich. Castor oil were obtained from Organic
Supply Co.
2.2 GC-MS Analysis of Castor Oil
(Ricinus communis)
Transesterification reaction castor oil was carried out
Stywarni, G., Iftitah, E. and Srihardyastutie, A.
Lactonization Castor Oil (Ricinus Communis) using Lipase B from Candida Antarctica Recombined Aspergillus oryzae as Bioflavor.
DOI: 10.5220/0009955000370040
In Proceedings of the 2nd International Conference of Essential Oils (ICEO 2019), pages 37-40
ISBN: 978-989-758-456-5
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
37

to determine the components of fatty acids. 50 g
castor oil, 38 mL of ethanol
and 1 mL of H
2
SO
4
1 M
were put in 100 mL
Erlenmeyer flasks. The mixture
was refluxed at 60-70ºC for 2 h. Then, Saturated NaCl
was added to separate organic and water phase. The
organic phase dehydrated by anhydrous
Na
2
SO
4
and
dissolved in n-hexane (1:40, v/v).
Gas Chromatography (GC) equipped with a Mass
Spectra (MS) detector and Restrex
Rxi®-1MS
capillary column. Oven temperature
was held at 40 -
250ºC; injection temperature was 250ºC; The carrier
gas, helium, was adjusted to a linear velocity 0.7
mL/min and 24.9 kPa. The injection volume
into the
GC apparatus was 0.5µl.
2.3 Castor Oil Lactonization
The r eact ion was carried out in 100 mL Erlenmeyer
flasks, containing 6 g castor oil,
40 mL of n-hexane
solvent,1 mL of Na
2
CO
3
solution, and 0.1 g Candida
antarctica recombined Aspergillus oryzae. The
reactions were stirred using magnetic hotplate stirrer
at room temperature and 40ºC for 24, 48 and 72 h.
Then the pH of the mixture was measured. Each
sample was centrifuged to separate the enzyme a n d
the oil phase. The samples dissolved in n-
hexane (1:20, v/v).
Gas Chromatography (GC) equipped with
a Mass Spectra (MS) detector and Restrex Rtx®-
5MS capillary column. Oven temperature w a s
held at 40 - 250ºC; injection
temperature
was
250ºC;
The
carrier
gas,
helium, was adjusted
to a linear velocity 1.01 ml/min and 50 kPa. The
injection samples into the GC apparatus was 0.5µl.
3 RESULTS AND DISCUSSION
3.1 Analysis of Castor Oil
Substrate (Ricinus communis)
Generally, the composition of ricinoleic acid in castor
oil comprises approximately 90%, while the
composition of other fatty acids: linoleic acid, oleic
acid, stearic acid, palmitic acid, dihydroxystearic
acid, linolenic acid, and eicosanoic acid less than
5% (Kourist and Hilterhaus, 2015). Based on
analysis using GC-MS, the highest % concentration
transesterification product castor oil was methyl
ricinoleate (88.666%) (Table 1). So that, ricinoleic
acid is the major component of castor oil. Some fatty
acid components were not found in castor oil
substrate, but % concentration of ricinoleic acid as
the major component was good quantity.
Table 1: Trans-esterification product of castor oil.
%
Cons.
Compound
0.872
Methyl-14-methyl
pentadecanoate
4.285 Methyl-9-12-
octadecadienoate
4.547 Methyl-11-
octadecenoate
1.630 Methyl octadecanoate
88.666 Methyl ricinoleate
2.4 Effect of Temperature and
Reaction Time
Lactonization using Candida antarctica lipase B
recombined Aspergillus oryzae not only produce
lactone. The lactone was γ- dodecalactone only
formed at room temperature for 48 h. (Table 2).
Table 2: Effect temperature and reaction time on lactone
formation.
Temperature
(ºC)
Reaction
Time (h)
Lactone
room
24 -
48
√
72 -
40
24 -
48 -
72 -
Biotransformation product of castor oil at room
temperature were esters, fatty acids and lactone.
Whereas at 40ºC only formed esters (Table 3).
Table 3: Biotransformation product of castor oil.
T
(ºC)
t
(h)
Compound % Area
Ambient
24 Methyl ricinoleate 53.64
48
9-octadecenoic acid 4.37
γ-Dodecalactone 1.75
Methyl dodecanoate 4.58
Dodecanoic acid 1.53
Methyl-9-
octadecenoat
1.59
72
Methyl dodecanoat 1.61
Methyl ricinoleat 12.77
40 24 Methyl ricinoleat 69.90
48 Methyl ricinoleat 64.95
72 Methil ricinoleat 81.33
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
38
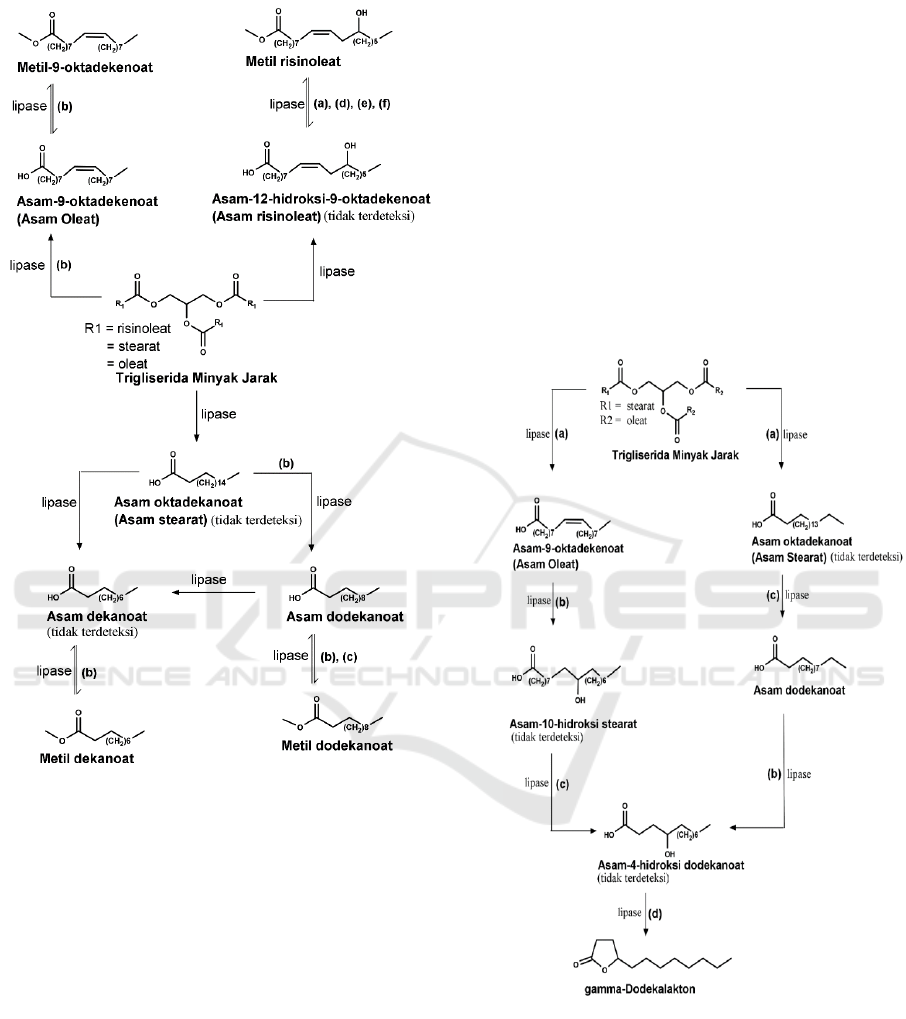
Figure 1. Estimated mechanism formation of fatty acid and
ester. Condition of reaction: (a)T= room, t = 24 h; (b)T=
room, t = 48 h; (c)T= room, T = 72 h; (d)T= 40ºC, t =24
h; (e)T= 40ºC, t = 48 h; (f) T = 40ºC, t = 72 h.
As the date in Table 3 show, Biotransformation of
castor oil at room temperature and 0ºC produces
esters (methyl ricinoleate) as the major product. It can
be assumed that before the esterification reaction,
triglyceride was hydrolysed to fatty acids, ricinoleic
acid (undetectable). The optimal yield of methyl
ricinoleate at 40ºC for 72 h (81.33%), is higher than
the methyl ricinoleate at room temperature.
Formation C
18
Fatty acid products at
room
temperature (9-octadecenoic acid, t =
48 h) and C
12
(
dodecanoic acid, t = 48 h)
showed that lipase B
Candida antarctica recombined Aspergillus oryzae
had ability to hydrolysis triglyceride and shortening
fatty acid carbon chain. This is probably hydrolysis
reaction because lipase had active side catalytic:
serine, histidine, and aspartate (Veld, 2010). The
shortening C
18
fatty acid chain to C
12
can be
assumed
at beta carbon position that occur oxidation into
carbonyl groups as much as three times. Source
water in hydrolysis
reaction form added Na
2
CO
3
solution
as
component reaction. Then, fatty acid was
probably forming ester. The fatty acid is then
probably transformed into ester. Formation reaction
of fatty acid and ester showed at Figure 1 9-
octadecanoic acid probably to form
methyl-9-
octadecanoic
(t = 48 h), dodecanoic acid probably to
form methyl dodecanoate (t = 48 and 72 h). Methyl
dodecanoate product (t = 72 h, 1.61%), lower than
methyl dodecanoate (t = 48 h, 4.58%).
Figure 2. Estimated mechanism formation of
gamma-dodecalactone. Reaction: (a) hydrolysis,
(b) hydroxylation (c) shortening of carbon chains
(d) lactonization.
Lactonization reaction to form γ- dodecalactone
is probably from 9- octadecanoic acid, then
hydroxylated to form 10-hydroxy octadecanoic acid
(undetectable), after that undergoes a carbon chain
Lactonization Castor Oil (Ricinus Communis) using Lipase B from Candida Antarctica Recombined Aspergillus oryzae as Bioflavor
39

shortening to form 4-hydroxy dodecanoic acid
(undetectable), and it was occurring lactonization
become γ-dodecalactone. Formation of γ-
dodecalactone product is also probably from
dodecanoic acid (Figure 2). The mechanism of γ-
dodecalactone formation (Figure 2) refers to Han et
al. (1995) who use Mortierella isabellina on
dodecanoic acid substrate and Haffner et al. (1996)
using the sporobolomyces odour on 9-octadecanoic
acid (oleic acid) that occur hydroxylation
(Goswami et al., 2013) to form γ-dodecalactone
The target compound that is γ-decalactone was
not formed in
biotransformation
of castor oil at room
temperature and 40ºC, it is estimated that due to
ideal biotransformation reaction conditions for
esterification, so that the hydroxylation reaction to
form ricinoleic acid (not detected) as substrate
probably convert quickly to ester (methyl
ricinoleate). The formation of another lactone (γ-
dodecalactone, 1.75%) as minor product at room
temperature for 48 h probably so because the
hydrolysis of 9-octadecenoic acid and dodecanoic
acid was formed during the 48 h, so that
lactonization (intra-esterification reaction) is
possible at these time.
4 CONCLUSIONS
Lactonization of castor oil only produces lactone at
room
temperature
for 48 h. The lactone product was γ-
dodekalakton as minor product (1.75%). The major
products biotransformation was methyl ricinoleat
(T=room, t=24 h: 53.64%); (T=room, t= 72 jam:
12.77%); (T=40ºC, t=24 h: 69.90%); (T=40ºC, t=48 h:
64.95%); (T=40ºC, t=72 h 81.33%).
REFERENCES
Antczak, U., Gora J., Antczak, T., Galas, E., 1991.
Enzymatic
Lactonization of
15-Hydroxypentadecenoic
and 16-Hyhroxyhexadecenoic Acids
to
Macrocyclic
Lactones. Enzyme Microbial Technology, 13, 589-593.
Farbood, M, I., Willis B, J., 1985. Production of ᵧ-
Decalactone. US Patent, 306, 691.
Goswami, D., Basu, J.K.,
De,
Sirhendu., 2013. Lipase
Applications
in
Oil Hydrolysis with a Case Study on
Castor
Oil:
A Review. Critical Reviews
in
Biotechnology, 33(1), 81–96.
Gotz, K., Liase, A., Ansorge-Schumacher M., Hilterhalus,
L., 2013. A- Chemo-Enzymatic Route to Synthesize
(S)-ᵧ- Valerolactone From Levulinic Acid. Application
Microbial Biotechnology, 97, 3865-3873.
Gutman, A, L., Zuobi, K., Bravdo, T., 1989. Lipase-
Catalyzed Preparation of Optically Active γ-
Butyrolactones in Organic Solvent. Journal Organic
Chemistry, 55, 3546-3552.
Khan, N, R., Rathod, V, R., 2018. Microwave Assisted
Enzymatis Synthesis of Specially Esters: A Mini -
Review. Process Biochemistry, Department of
Chemical Engineering, India, Institute of Chemical
Technology.
Kourist, R., Hilterhaus, R., 2015. Microbial Lactone
Synthesis Based on Renewable Resources,
Microbiology Monographs, 2, 277-278.
Moradi, H., Asadollahi, M, A., Nahvi, I., 2013. Improved
γ-Decalactone Production from Castor Oil by Fed-
Batch Cultivation of Yarrowia Lipolytica. Biocatalytic
Agriculture Biotechnology, 2(1), 64–68.
Wache, Y., Aguedo, M., Choquet, A., Gatfield, IL., Nicaud,
J-M., Belin, J-M., 2001. Role of Beta-Oxidation
Enzymes in Gamma-Decalactone Production by the
Yeast Yarrowia Lipolytica, Applied And
Environmental Microbiology, 67(12),
5700–5704.
Veld, M, A, J., 2010. Candida antarctica Lipase B catalysis
inorganic, polymer and supramolecular
chemistry:Eindhoven University of Technology.
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
40
