
FRADE: Pervasive Platform for Fall Risk Assessment,
Prevention and Fall Detection
Joana Silva
a
, Nuno Cardoso
b
, Jorge Ribeiro
c
, Alberto Carvalho
d
, Mariana Pereira
e
,
Fernando Ricaldoni
f
, Carlos Resende
g
and Jo
˜
ao Oliveira
h
Fraunhofer Portugal Research Center for Assistive Information and Communication Solutions,
Rua Alfredo Allen 455/461, 4200-135 Porto, Portugal
Keywords:
Fall Detection, Fall Risk Assessment, Fall Prevention, Otago Program, Inertial Sensors, Wearable Device.
Abstract:
The ageing of the global population has an impact on the elderly quality of life, as the reduced mobility and
balance contribute to the increasing of falls. Fall detection solutions can trigger emergency alerts and reduce
the negative effect of falls. Fall risk assessment strategies can help to early identify fall risk factors and tailor
strategies to revert those risk factors, by means of fall prevention exercises. However, most technological solu-
tions do not simultaneously address these three aspects of the falls management cycle. FRADE platform will
allow to pervasively detect falls using a wearable device, that can also be used to monitor fall risk assessment
tests and recommended individual exercises, that can be performed at home with a tablet and two wearables.
1 INTRODUCTION
The worldwide population aged over 65 is rapidly
growing and the consequences are simultaneously so-
cial, health-related and economic. The process of
ageing impacts mobility, muscle strength and balance
control which contributes to the increase of falls oc-
currence in this population. Currently, there are a va-
riety of solutions to address only specific stages of
the fall management lifecycle: assessing multiple fall
risk factors, detecting falls automatically, and provid-
ing strategies for falls prevention that focus on atten-
uating specific fall risk factors (Rajagopalan et al.,
2017). However, most technological solutions do not
allow to close the falls management loop by simul-
taneously addressing fall detection (FD), fall risk as-
sessment (FRA) and prevention (FP).
Among the elderly population, falls are one of the
major causes of death and injury. More than 30% of
people over 65 falls each year and the prevalence in-
a
https://orcid.org/0000-0002-6214-5868
b
https://orcid.org/0000-0001-5736-7995
c
https://orcid.org/0000-0001-5532-2574
d
https://orcid.org/0000-0002-1458-687X
e
https://orcid.org/0000-0002-3278-1230
f
https://orcid.org/0000-0003-2836-8782
g
https://orcid.org/0000-0002-1834-0420
h
https://orcid.org/0000-0003-3667-4958
creases for people above 80 (Bergen et al., 2016). Be-
sides social and personal consequences, falls also play
an important role in healthcare costs. Centers for Dis-
ease Control and Prevention estimate approximately
645 fall-related emergency visits for every 10000 el-
derly. In the USA in 2015, the direct costs for fatal
and nonfatal fall injuries were 637,5 million and 31,3
billion dollars, respectively (Burns et al., 2016). The
Personal Emergency Response Systems (PERS) mar-
ket is valued at 6245 million in 2018 and expected
to reach 9452 million dollars by 2025 (Bergen et al.,
2019).
Even a minor fall can severely affect the physi-
cal and mental health of an elder and increase the
fear of falling again. Thus, the elderly quality of
life and of their caregivers can be severally affected.
For community-dwelling elderly, the problem affects
mostly the elder and his family. However, given the
high demand for specialized care due to aging and
falls, most of the older adults need to be institution-
alized, and thus the problems also affect daycare cen-
ters, retirement homes, nursing homes, and healthcare
facilities. Fall detection solutions can trigger emer-
gency alerts and reduce the negative effect of falls.
FRA strategies can help to early identify fall risk and
tailor strategies to revert those risk factors.
Currently, there are a variety of solutions to ad-
dress only specific stages of the fall management cy-
cle: assessing multiple fall risk factors, detecting falls
320
Silva, J., Cardoso, N., Ribeiro, J., Carvalho, A., Pereira, M., Ricaldoni, F., Resende, C. and Oliveira, J.
FRADE: Pervasive Platform for Fall Risk Assessment, Prevention and Fall Detection.
DOI: 10.5220/0010328103200326
In Proceedings of the 14th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2021) - Volume 4: BIOSIGNALS, pages 320-326
ISBN: 978-989-758-490-9
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

automatically, and providing strategies for falls pre-
vention that focus on attenuating specific fall risk fac-
tors. However, current solutions fail to provide a com-
plete system for fall detection, fall risk assessment,
and fall prevention, which would enable value-based
healthcare. Most commercial solutions available in
the market target only one specific functionality.
Personal emergency response systems (PERS), or
medical alert services, provide prompt access to emer-
gency help for elderly who fall. PERS are usually
in the form of a pendant or wrist band, and enable
the users to press a button, to transmits a signal to
a call center or caregiver, who contacts emergency
help. Most PERS are not automatic, instead, the user
must press a button. PERS are stigmatizing since they
should be attached to a specific on-body position, and
most of them do not allow the user to select differ-
ent levels of sensitivity for FD. The main competitors
are Philips Lifeline
1
, Medical Guardian
2
, AlertOne
Services
3
, Tunstall
4
and Life Alert Emergency Re-
sponse
5
. These companies provide PERS with an
emergency button, but few detect falls automatically.
FRA solutions fail to address multiple risk fac-
tors and only provide an estimation of FRA based
on a limited number of risk factors evaluated in spe-
cific periods. There are several commercial solu-
tions for FRA that can use wearable devices or pres-
sure sensors to evaluate standard tests, such as QTUG
from Kinesis
6
, Go from GaitUp
7
and MoveTest from
McRoberts
8
.
FP solutions do not combine the assessment of
fall risk factors with personalization of FP strate-
gies. There are a variety of solutions that provide
technological support to gamify physiotherapy exer-
cises, either with wearable devices, pressure sensors
or Kinect, such as Sword Health
9
, Biosensics
10
and
SilverFit
11
.
Considering that there are no existing solutions
that close the falls management loop, FRADE will
be unique in that perspective. Such level of integra-
tion comes with benefits in the acquisition and main-
1
https://www.lifeline.philips.com/medical-alert-systems/
fall-detection.html
2
https://www.medicalguardian.com/
3
https://www.alert-1.com/
4
https://www.tunstall.co.uk/resources/product-datasheets/
vibby-fall-detector/
5
http://www.lifealert.com/
6
https://www.kinesis.ie/qtug/
7
https://clinical.gaitup.com/gait-up-go/
8
https://www.mcroberts.nl/products/movetest/
9
https://swordhealth.com/
10
https://biosensics.com/
11
https://silverfit.com/en/
tenance costs, which can be cheaper than acquiring
individual solutions. It also brings a broader view of
the elder status, where all the information is available
in a single platform, which should improve the effi-
ciency of healthcare for the elderly population. The
FD system is a standalone wearable device that sup-
ports multiple body positions. The FRA is composed
of a multifactorial set of questionnaires and instru-
mented functional tests, which allow recommending
individual exercises.
2 FRADE PLATFORM
FRADE is composed of a bundle of components
(wearable sensor, desktop application, Android appli-
cation, data storage, and data visualization web in-
terface) to perform FD, FRA, and FP. The elder will
use the wearable sensor to monitor falls automatically,
based on movement analysis. It sends an alarm to the
backend server and an SMS to a caregiver whenever
a fall is detected. The wearable device will also be
used to monitor the elder’s movements and communi-
cate with the desktop and Android application while
he performs FRA tests and FP exergames. All the
monitored data is stored in the data storage and can
be accessed through a web interface, by a caregiver or
an healthcare provider.
2.1 Fall Detection Wearable Device
Kallisto is an hardware module with sensing, com-
munication and power management capabilities. It
includes a set of inertial and ambient sensors, Blue-
tooth Low Energy (BLE) and NFC radios, as well as
USB and Qi inductive charging systems. It is supplied
as a module and can be used as standalone or as part
of a more complex device. To build the wearable de-
vice, the COMM mainboard was used that comprises
Kallisto as its core block and complements the sys-
tem by providing extra sensing and communication
functionalities. It includes GNSS, Narrow Band-IOT
(NB-IoT) and RFID radios
12
.
The wearable device casing was conceived from a
user-centered perspective in a way of making it easy
to use whilst accommodating the HW platform. The
design approach was to make the device almost part
of the user clothes, as a simple shirt button for exam-
ple, as represented in Figure 2. When attached to the
clothes the device will provide automatic fall detec-
tion, and occasionally, when attached on an accessory
to the users’ thighs or feet, it will provide data stream
for movement analysis.
12
https://demo.sensry.net/
FRADE: Pervasive Platform for Fall Risk Assessment, Prevention and Fall Detection
321
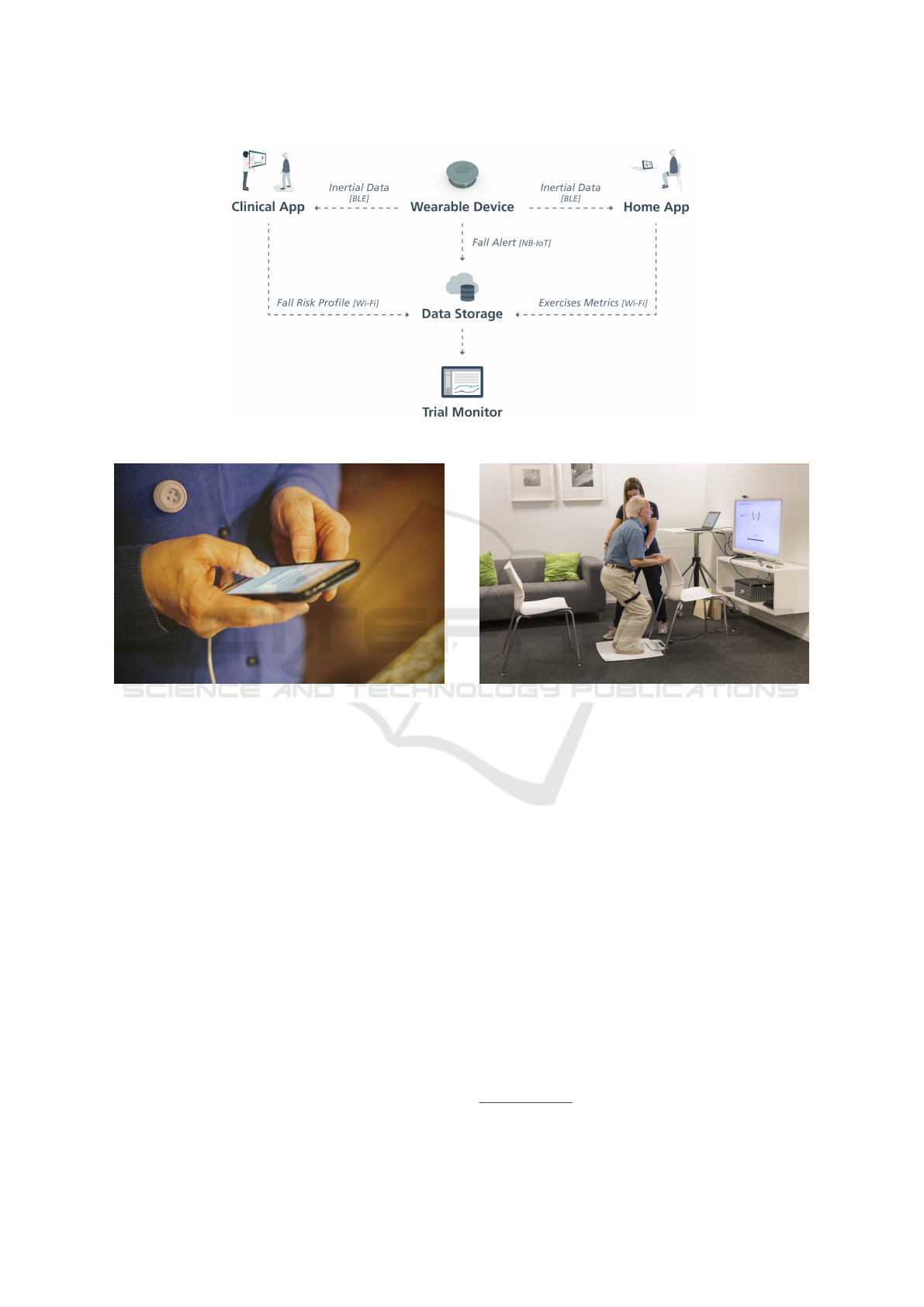
Figure 1: Hardware and software components of the FRADE platform.
Figure 2: Wearable device Kallisto placed in the clothes as
a button.
This wearable device integrates an automatic fall
detection algorithm and offers features such as alarm
sending, through the wearable device, which can be
used discreetly by the user in the belt, pocket or in the
chest. The fall detection algorithm is embedded in the
firmware of the wearable device. This algorithm anal-
yses the inertial sensors data and detect whenever a
fall incident occurs, as previously described in (Alves
et al., 2019). In case of detection of a fall event, a noti-
fication will be sent to a data storage that in turn sends
an SMS and e-mail to a set of predefined emergency
contacts. The communication between the device and
the data storage is made through NB-IoT. The de-
vice also triggers an audible alarm to attract the at-
tention of people nearby, featuring an emergency but-
ton that allows the cancellation of false alarms. The
device works independently of a smartphone or other
resource and only needs to be charged via an induc-
tion charger, which is made available with the device.
Figure 3: Elderly performing the fall risk screening using
the Clinical App.
2.2 Fall Risk Assessment Clinical
Application
The Clinical app is a Windows desktop application
that was designed for the healthcare providers to al-
low the creation of questionnaires and functional tests
to screen the elderly regarding the fall risk. The exer-
cise monitoring algorithms are based on the analysis
of movement and balance (Martins et al., 2018) using
sensor data from two wearable devices and a Phys-
ioSensing pressure platform, as can be seen in Figure
3. This application also allows healthcare providers
to prescribe exercises for the home application. The
application was developed in Unity 2019.3.3 and re-
quires a laptop with BLE to connect to the wearable
devices and a USB port to connect to the pressure
platform
13
.
The Clinical App extracts information about the
movement and balance of the user while he is per-
13
https://www.physiosensing.net/
BIOSIGNALS 2021 - 14th International Conference on Bio-inspired Systems and Signal Processing
322

forming the functional tests Sit-to-Stand Test (Cho
et al., 2012), Timed-Up and Go Test (Kojima et al.,
2015), and 4 Stage Balance Test (Thomas et al.,
2014), as shown in the main screen of Figure 4. The
application also allows the insertion of the personal
profile of each participant and the answer to several
questionnaires for the assessment of several risk fac-
tors for falls, such as activities of daily living (Ara
´
ujo
et al., 2008), fear of falling (Figueiredo and Santos,
2017), and home hazards (Watson et al., 2014) ques-
tionnaires. In addition, the application allows the pre-
scription of Otago Exercise Program (OEP) exercises
and their schedule for each participant through a ded-
icated exercise prescription interface. This exercise
prescription will be sent automatically to the Home
application.
Figure 4: Screening exercises in the Clinical App.
2.3 Fall Prevention Home Application
The Home App is an Android tablet-based application
for the elderly to perform fall prevention exercises
based on the Otago Programme, an evidence-based
program for reducing falls (Campbell et al., 1997).
The application provides interfaces with instructions
on how to perform the exercises, as in Figure 5, and
interfaces with feedback during the exercises, which
are based on the movement analysis algorithms pro-
vided by the inertial sensors of a wearable device, as
previously described in (Silva et al., 2018).
The interactive Home application, based on the
Otago exercise program, aims to improve physical
functionality. The application features 8 exercises
from the program, i.e., knee flexion, knee extension,
hip abduction, knee bending, toe raises, calf raises, sit
to stand, and one leg standing, that are static, easy and
well accepted exercises with interactive feedback on
the execution of the movement, as depicted in Figure
6. The application could also be a source of moti-
vation for the participants who perform the exercises
at home. The use of the application requires an An-
droid Tablet, a support for the tablet, two wearable
inertial devices, and the respective chargers. The ap-
plication only requires internet connectivity once at
the app setup and occasionally to synchronize the ex-
ercises metrics with the cloud (on follow up appoint-
ments with the clinician). The application is compat-
ible with any tablet with Android version above An-
droid 4.4 (API 19). The user will be guided through
a weekly exercise plan, which he/she will be able to
select through the tablet interface where the instruc-
tions for executing each exercise will be presented, as
well as an interface with visual feedback during the
execution of each exercise.
Figure 5: Example of one interface of the Otago exercises
using the Home App.
Figure 6: Execution of one exercise using the Home App.
2.4 Data Storage
All the information collected through the aforemen-
tioned components is stored on Firebase Cloud Fire-
store. Cloud Firestore is a non-relational database that
enable developers to safely store and sync data across
multiple devices and applications. Firebase is certi-
fied under major privacy and security standards and
follows the GDPR rules. All the data in Firebase is
also encrypted on the tablet side. Cloud Firestore is
also used to manage the authentication of users with
the home and clinical applications. Only registered
users can have access to the data, i.e., the healthcare
FRADE: Pervasive Platform for Fall Risk Assessment, Prevention and Fall Detection
323

providers or caregivers. Data from the clinical app,
home app, as well as from the wearable device are
stored on the database to be made available to the
other applications in the system (Fig. 1). For in-
stance, plans generated on the clinical app by health-
care providers are available for the elderly to follow
in the mobile app. Cloud Firestore stores the profile
data, such as height or clinical condition, the answers
to the questionnaires for risk assessment, plans cre-
ated by the healthcare providers, and session and ex-
ercise data detailing the measurements of individual
exercises. Besides allowing developers to synchro-
nize data across applications, it enable us to monitor
the elderly during the trials, and to keep a detailed log
of participants measurements for further analysis of
the trials results.
2.5 Trial Monitor
Trial Monitor is a web application created with the
purpose of supporting researchers monitoring partici-
pants remotely during field trials (Vasconcelos et al.,
2019). The tool enables researchers to create per-
sonalized data visualization dashboards with the data
generated by participants during the trials (Fig. 7).
The web application connects to the data storage (i.e.
Cloud Firestore) to retrieve users data, and generates
visualizations for health providers to follow the el-
derly remotely.
The platform enables nursers to understand how
participants are progressing during the trial, by pro-
viding a system for monitoring the results of the
prescribed exercises and how they evolve over time.
Healthcare providers can use the platform to visual-
ize and analyse the results of the Otago exercises, or
the fall risk assessment questionnaires. The platform
displays the number of sessions completed and the re-
sults from individual exercises (e.g. number of repe-
titions, range of motion). Moreover, the platform al-
lows researchers to easily export data (i.e. CSV file)
from the system for further analysis after the end of
the trial.
3 VALIDATION TRIALS
We have produced 20 units of system to be used dur-
ing the validation trials. The participants are being
recruited. The trials to be conducted will allow to
evaluate the performance of the FD and to assess the
fall risk of the elder population in the North region of
Portugal. The trials will also provide insights on the
effectiveness of the FP exercises using this technolog-
ical platform.
The study will bring the objective measure of fall
risk factors and movement-based metrics extracted
during the Otago exercises. The technological plat-
form allows the centralization of all relevant variables
in a unified and secured database, that is accessed
through a web portal, that will be available for the
healthcare providers that will supervise the study. Be-
sides the aforementioned variables retrieved by the
Clinical app, i.e., personal profile, medical conditions,
medication, answer to the questionnaires, and scores
of the three functional tests, the Home app will allow
to measure range of motion, number of repetitions and
duration of ascending and descending movements for
eight exercises of the OEP, i.e., knee flexion, knee ex-
tension, hip abduction, knee bending, toe raises, calf
raises, sit to stand, and one leg standing.
4 CONCLUSION
The use of technological devices for early detection
and prevention of falls is a key strategy to minimize
the consequences of this event, namely the perma-
nence on the ground for long periods after the fall and
hypothermia, which often leads to the death of the el-
derly. This reality is verified not only in the elder’s
home but also in nursing homes. To respond to this,
the scientific production in this area has been prolifer-
ating, both in the technological sector and in the aca-
demic and clinical context. The synergies created by
the joint work of these areas, allow this project to con-
tribute to the development of more effective strategies
for prevention, reduction of falls and their physical,
psychological and economic consequences.
In technological terms, we consider that the tech-
nological literacy of each participant could impact the
way each one will use the platform and we are aware
that some limitations may raise during the course of
the eight weeks of intervention, considering that in
some of the sessions the elderly will perform the ex-
ercises alone at home. To try to overcome these
limitations, we foresee frequent contacts between the
healthcare providers and the elderly, as well as remote
guidance whenever possible.
At the end of the validation study, we will have
a system for fall management composed of a wear-
able device and a bundle of applications. The system
can be used to i) pervasively detect falls and assess
fall risk factors in daily life; ii) monitor movements
during fall risk assessment tests to estimate a risk of
falling using a desktop application, with the supervi-
sion of a healthcare provider; iii) monitor movements
during the execution of fall prevention exercises us-
ing by a tablet application. The trials conducted will
BIOSIGNALS 2021 - 14th International Conference on Bio-inspired Systems and Signal Processing
324

Figure 7: Trial monitor web platform displaying the metrics of the Otago exercises.
allow us to evaluate the performance of the FD, and
to screen part of the institutionalized population in the
North region of Portugal. The FD, FRA will provide
insights on the effectiveness of the fall prevention ex-
ercises.
This validation study aims to evaluate the effec-
tiveness of the technological solution for detecting
falls and the identification of the risk of falling in the
elderly at their homes, as well as validating a tech-
nological solution for fall prevention. The primary
economic buyer will be companies that sell healthcare
equipment, mainly to nursing homes and daycare cen-
ters. The secondary economic buyer will be the nurs-
ing homes and healthcare institutions and the tertiary
economic buyer will be the final consumer, the elderly
since the solution can also fit a home scenario.
The main obstacles for our solution to reach the
market are mainly related to the user and market ac-
ceptance. The technological solutions that will be in-
tegrated into this system were previously developed
based on user-centered design and all the prototypes
were tested with the elderly in usability tests using a
network of end-users and institutions. The fact that
the same wearable can be used for FD, FRA and FP,
will require less devices to be bought and maintained.
The same applies to the monthly fees normally associ-
ated with these services, there will be a single fee for
a system that covers all components, instead of a fee
for each component. We can also mitigate the risks
of poor market acceptance by providing only parts of
the system.
ACKNOWLEDGEMENTS
The authors would like to thank all the participants
involved in the usability tests, and the healthcare
providers that collaborated in the definition of the re-
quirements for the technological solution. Authors
would like to thank the financial support of Project
RIS-1001-8273 of EIT Health RIS Innovation Call
2020; EIT Health is supported by the EIT, a body of
the European Union.
REFERENCES
Alves, J., Silva, J., Grifo, E., Resende, C., and Sousa, I.
(2019). Wearable embedded intelligence for detection
of falls independently of on-body location. Sensors,
19(11).
Ara
´
ujo, F., Ribeiro, J., Oliveira, A., Pinto, C., and Martins,
T. (2008). Validac¸
˜
ao da escala de Lawton & Brody
numa amostra de idosos n
˜
ao institucionalizados. Actas
do 7
o
Congresso Nacional de Psicologia da Sa
´
ude,
pages 217–220.
Bergen, G., Stevens, M., and Burns, E. (2016). Falls and fall
injuries among adults aged > 65 years - United States,
2014. MMWR. Morbidity and mortality weekly report,
65:993–998.
Bergen, G., Stevens, M., and Burns, E. (2019). Global
personal emergency response systems (PERS) market:
Global market size, trends, competitive, historical &
forecast analysis, 2019-2025. BMRC 854,, Medical
Devices and Consumables:202.
Burns, E. R., Stevens, J., and Lee, R. (2016). The direct
costs of fatal and non-fatal falls among older adults -
United States. Journal of safety research, 58:99–103.
Campbell, A. et al. (1997). Randomised controlled trial of
a general practice programme of home based exercise
to prevent falls in elderly women. BMJ (Clinical re-
search ed.), 315:1065–9.
Cho, k. H., Bok, S., Kim, Y.-J., and Hwang, S. (2012). Ef-
fect of lower limb strength on falls and balance of the
elderly. Annals of rehabilitation medicine, 36:386–93.
Figueiredo, D. and Santos, S. (2017). Cross-cultural val-
idation of the falls efficacy scale-international (FES-
FRADE: Pervasive Platform for Fall Risk Assessment, Prevention and Fall Detection
325

I) in portuguese community-dwelling older adults.
Archives of gerontology and geriatrics, 68:168–173.
Kojima, G., Masud, T., Kendrick, D., Morris, R., Gawler,
S., Treml, J., and Iliffe, S. (2015). Does the timed
up and go test predict future falls among british
community-dwelling older people? prospective co-
hort study nested within a randomised controlled trial.
BMC Geriatrics, 15.
Martins, A., Moreira, J., Silva, C., Silva, J., Tonelo, C., Bal-
tazar, D., Rocha, C., Pereira, T., and Sousa, I. (2018).
Multifactorial screening tool for determining fall risk
in community-dwelling adults aged 50 years or over
(fallsensing): Protocol for a prospective study. JMIR
Research Protocols, 7.
Rajagopalan, R., Litvan, I., and Jung, T.-P. (2017). Fall
prediction and prevention systems: Recent trends,
challenges, and future research directions. Sensors,
17(11).
Silva, J., Moreira, D., Madureira, J., Pereira, E., Dias, A.,
and Sousa, I. (2018). A technological solution for sup-
porting fall prevention exercises at the physiotherapy
clinic. 2018 IEEE International Symposium on Medi-
cal Measurements and Applications (MeMeA).
Thomas, J. C., Odonkor, C., Griffith, L., Holt, N., Percac-
Lima, S., Leveille, S., Ni, P., Latham, N. K., Jette,
A. M., and Bean, J. F. (2014). Reconceptualizing bal-
ance: attributes associated with balance performance.
Experimental Gerontology, 57:218 – 223.
Vasconcelos, A., Lopes, I., Ribeiro, J., and Barros, A. C.
(2019). Challenges and lessons learned from imple-
menting longitudinal studies for self-care technology
assessment. LDC 2019: Workshop on Longitudi-
nal Mobile, Wearable and Ubiquitous Data Collection
from Human Subject Studies, pages 893–898.
Watson, M., Benford, P., Coupland, C., Clacy, R., Hind-
march, P., Majsak-Newman, G., Deave, T., and
Kendrick, D. (2014). Validation of a home safety
questionnaire used in a series of case-control studies.
Injury Prevention: Journal of the International Soci-
ety for Child and Adolescent Injury Prevention, 20.
BIOSIGNALS 2021 - 14th International Conference on Bio-inspired Systems and Signal Processing
326
