
Design Approach of Medical Devices for Regulation Compatibility:
A Robotic Rehabilitation Case Study
Raffaele Formicola
1a
, Federica Ragni
1b
, Maurizio Mor
2c
,
Luciano Bissolotti
3d
and Cinzia Amici
1e
1
Department of Industrial and Mechanical Engineering, University of Brescia, via Branze 38, 25123, Brescia, Italy
2
Polibrixia s.r.l., via Branze 43, 25123, Brescia, Italy
3
Laboratorio Larin, Casa di Cura Domus Salutis, Fondazione Teresa Camplani, via Lazzaretto 3, 25123, Brescia, Italy
Keywords: Design Process, Bio-Medical Devices, MEDDEV, Regulations, CE Mark, Robotic Rehabilitation, Design
Requirements, Bio-Compatible Materials, Selection Criteria.
Abstract: Regulations and normative framework strongly affect requirements and potential design constraints of
devices, especially in critical environments like the medical field, characterized by a complex interaction
among design, therapy procedures and user needs. In order to optimize the design process, the awareness of
the designer about the compound information net generated by the required documentation becomes therefore
fundamental. Depicting a custom mapping of required data and referring documents for the development and
commercialization of a medical device as required by the Conformité Européenne (CE) marking process, this
paper presents a design approach directly suitable for robotic rehabilitation systems, which aims at easing the
regulations compatibility of the designed product. This method is applied to the illustrative case study of the
LEPRE (LEg Programmable REhabilitation) robotic system, with particular attention to data collection and
analysis for the evaluation of clinical background and demonstration of equivalence required by the device
clinical evaluation report, according to MEDical DEVices (MEDDEV) 2.7/1 guidelines. Indications for the
modifications required to adapt it to further application fields are also suggested.
1 INTRODUCTION
Health technologies such as medical devices are
essential to guarantee good health care and the aging
well possibility, and technological innovations in
prevention and rehabilitative fields are gaining
importance in funded research (Amici et al., 2016).
The medical, and in particular the rehabilitative,
environment involves a complex interaction among
design, user needs, and therapy procedures
(Hagedorn et al., 2015). Considering the possible
design methods for medical devices, literature
suggested that both physician and engineer
participate in the design phase of a rehabilitative
device (Amici et al., 2016) since the earlier design
phases: the physician defines the functional
a
https://orcid.org/0000-0002-5526-0155
b
https://orcid.org/0000-0002-4186-5356
c
https://orcid.org/0000-0001-5840-3636
d
https://orcid.org/0000-0002-5882-645X
e
https://orcid.org/0000-0001-7426-6029
requirements of the system (i.e. maximum force or
acceleration needed, movement to reproduce with the
device), whereas the engineer develops the technical
specifications of the device (i.e. kind of actuation, or
structure). Then, the final user can support the process
providing feedbacks along the development path.
The necessity of ensuring the patient benefit and
safety led many countries to introduce regulatory
instruments (Henschke et al., 2016) to demonstrate
and guarantee safety and effectiveness of devices to
be launched on the market (Kramer et al., 2020;
Römer and Stuyt, 2007). Given the fundamental role
of regulations in this process (Privitera et al., 2017),
considering them from the earliest stages of the
device design and project management should be
good practice for manufacturers.
146
Formicola, R., Ragni, F., Mor, M., Bissolotti, L. and Amici, C.
Design Approach of Medical Devices for Regulation Compatibility: A Robotic Rehabilitation Case Study.
DOI: 10.5220/0010402801460153
In Proceedings of the 7th International Conference on Information and Communication Technologies for Ageing Well and e-Health (ICT4AWE 2021), pages 146-153
ISBN: 978-989-758-506-7
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

In the European Union (EU), medical devices can
be marketed across all EU member states after
earning the Conformité Européenne (CE) mark; that
guarantees streamlined trade, safety, and
environmental standards (Council of the European
Union, 1993), and defines essential requirements and
recommendations concerning clinical evaluation and
vigilance (Council of the European Union, 2007).
To obtain the CE mark, the MEDical DEVices
(MEDDEV) set of documents (European
Commission, 2016) is a helpful instrument, since
represents non-binding guidance, which deals with
the application of the directives on medical devices,
such as consensus statements and interpretative
documents (Fraser et al., 2011). Besides, since the
clinical evaluation has become relevant for
manufacturers ((Council of the European Union,
1993) consistent with (European Parliament; Council
of the European Union, 2017)), the MEDDEV
documents state the guidelines to correctly perform
the clinical evaluation of a medical device and sum-
marize it in the Clinical Evaluation Report (CER).
According to MEDDEV 2.7/1, the CER document
includes, among others, two sections: i) clinical
background, and ii) demonstration of equivalence.
The first part produces as main output a systematic
review of the current knowledge in the medical field
of interest. Instead, the demonstration of equivalence
can be fulfilled through different possible methods: a)
comparing the new device with an equivalent
certified device, b) collecting clinical evaluations to
certify safety, performance, design characteristics,
and intended purpose of the device, or c) thanks to a
combination of these two options. Among the
considered characteristics, the evaluation of clinical,
technical, and biological factors is specifically
required.
In this complex scenario, the design of
rehabilitative devices should therefore consider not
only technical but also normative requirements,
which could introduce not negligible constraints since
the device conceiving phase. This work presents an
integrated design approach for medical devices,
which aims at easing the regulations compatibility of
the designed product, applied to the illustrative case
study of the LEPRE (LEg Programmable
REhabilitation) robotic system (Amici et al., 2019).
2 MATERIALS AND METHODS
LEPRE (PoliBrixia, Italy) is an end-effector based
robotic device for limb rehabilitation and is
characterized by two degrees of freedom that allow
the implementation of every motion profile in the
desired plane (Ceresoli et al., 2019). Within the
current normative framework, the CER of this device
was performed according to MEDDEV 2.7/1.
2.1 Interpretation of the Normative
Framework
According to the MEDDEV 2.7/1 guidelines, the
CER collects information from several areas of
knowledge. Some of the required data partially
overlap the informative content of other documents,
mandatory as well for the CE mark earning. For
instance, much of the information required in the
device risk assessment is also stated in the risk
analysis document (International Organization for
Standardization, 2019) of the manufacturing
company. For this reason, an analysis of the whole
documentation regarding the device and the quality
system of the manufacturing company (International
Organization for Standardization, 2016) was
performed, looking for consistency between data
required according to MEDDEV 2.7/1 indications
and already existing documents, with the final aim of
optimizing the clinical evaluation process. According
to this custom analysis, a mapping of required data
and referring documents was then performed.
2.2 Clinical Background
The bibliographic research was conducted following
the PICO systematic review strategy
(Patient/Problem, Intervention, Comparison,
Outcome) (Schardt et al., 2007) using as the main
database PubMed (National Center of Biotechnology
Information), and then extended to the ClinicalTrials
(Clinical Trials) and Cochrane (Cochrane Library)
databases. The optional fields Comparison and
Outcome of the PICO technique were omitted. Table
1 collects the selected keywords for the
Patient/Problem and the Intervention fields.
The identified keywords were combined in 5
search strings: s1) neurological AND (robotic AND
rehabilitation); s2) orthopaedic AND (robotic AND
rehabilitation); s3) rehabilitation AND robot-assisted;
s4) upper-limb AND (robotic AND rehabilitation);
s5) lower-limb AND (robotic AND rehabilitation).
For each string and each database, an independent
query was performed.
Inclusion criteria for the selection of the
documents were: i) document type Review or
Systematic Review; ii) document language English or
Italian; iii) publication date between 01.01.1990 -
01.06.2020. Exclusion criteria were: i) references that
Design Approach of Medical Devices for Regulation Compatibility: A Robotic Rehabilitation Case Study
147

are not relevant in terms of population/reference
pathology, ii) non-complete references, iii) too
general references, iv) references without a real
scientific contribution, v) duplicate references. No
further restrictions are reported.
To assure the consistency of the query among the
databases, the search strings were researched in all
fields for PubMed and Cochrane databases. In the
ClinicalTrials database, the query was implemented
by assigning to the “Condition or disease” mask field
the keywords search string of the PICO’s P field, and
to the “Other terms” mask field the keywords search
string of the PICO’s I field. For the Cochrane
database, only intervention documents were selected.
Queries were last updated on the 7
th
of July 2020.
The results of each query (five search strings for
three databases) were filtered excluding duplicates
and ordered by date (from newest records). Within the
results of each query, the first ten products were
selected, and the final set of documents was then
analyzed by a trained operator. According to
MEDDEV 2.7/1, a quantitative evaluation of the
documents has been performed, considering four
parameters: p1) publication date; p2) accordance
with search string; p3) reference population; and
p4) scientific consistency of the obtained results. For
each of the defined parameters, the operator assigned
a numerical value from 0 to 5 (0 not applicable, 5 fully
consistent for the search), and for each document, a
final score was computed as the sum of all the
parameters’ scores. Table 2 collects a synthesis of the
guidelines for the parameters’ score assignation. Two
evaluators, experienced in clinical and technical
context respectively, further checked the
reasonableness of the results. Only documents
presenting a final score higher than 14 were then
selected and considered as significant for the clinical
background.
2.3 Demonstration of Equivalence
For the LEPRE clinical evaluation, a free market
analysis was performed to find potentially equivalent
devices. Once identified the commercial names of
those devices, further bibliographic research was
conducted within the previously presented databases,
to extract the relevant scientific literature currently
available related to safety, performance, design
characteristics, and intended purpose of the devices.
The information gathered from those documents was
then integrated with the data available on the websites
of the manufacturers of the potentially equivalent
devices. The information gathered from those
documents was then integrated with the data available
Table 1: Selected keywords for PICO’s P and I fields.
Patient/Problem fiel
d
Intervention fiel
d
neurologic, orthopaedic,
rehabilitation, upper-limb,
lowe
r
-limb
robotic rehabilitation,
robot-assisted
Table 2: Guidelines for the parameters’ score assignation.
[0-5](points) assignation strateg
y
Parameter
p1
5: document (published or) updated in
2020
4: document updated in 2019
3: document updated in 2018
2: document updated between 2017-2015
1: document updated between 2014-2010
0: otherwise
p2
5: document very strongly related to the
search string requirements
4: document strongly related to the search
string requirements
3: document moderately related to the
search string requirements
2: document weakly related to the search
string requirements
1: document very weakly related to the
search string requirements
0: document not related to the search string
requirements
p3
5: document with very generic sample of
pathologic subject
4: document with generic sample of
pathologic subject
3: document with moderate generic
sample of pathologic subject
2: document with specific sample of
pathologic subject
1: document with very specific sample of
pathologic subject
0: document with not well-defined sample
of pathologic subject
p4
5: document with very strong theoretical
and practical importance results
4: document with strong theoretical and
practical importance results
3: document with moderate theoretical and
practical importance results
2: document with weak theoretical and
practical importance results
1: document with very weak theoretical
and practical importance results
0: document with no theoretical and
p
ractical im
p
ortance results
on the websites of the manufacturers of the
potentially equivalent devices.
According to MEDDEV 2.7/1 indications, the
biological equivalence was omitted, since the user is
strictly required to wear gloves or socks when using
ICT4AWE 2021 - 7th International Conference on Information and Communication Technologies for Ageing Well and e-Health
148

the LEPRE device, therefore no direct user-machine
contact is necessary.
3 RESULTS
3.1 Interpretation of the Normative
Framework
Figure 1 maps the required documents for the CE
marking process. Within the scheme, an example of
the data content interaction is provided, for the
specific case of LEPRE CER. Documents containing
the description of the device present a yellow dot,
whereas data regarding the demonstration of
equivalence are indicated with the green dot. The blue
dot depicts risk analysis-related data, and the red dot
is adopted for post-market surveillance information.
This scheme represents a first level simplification of
the data content interaction among documents sharing
information with CER. For each document within the
dashed boxes, further connections could be also
identified.
3.2 Clinical Background
After the selection process, 37 documents emerged
from the analysis of the PubMed, ClinicalTrials and
Cochrane databases. Table 3 synthesizes overall
results and post-filtering selected documents with
respect to databases and search strings.
Comparing the results of the five queries
performed within each database, 8 duplicated
documents emerged for PubMed, 7 for ClinicalTrials
and 28 for Cochrane, equal to 16,0%, 18,9% and
56,0% of the considered results for the specific
database, respectively.
Figure 2 depicts the trend of the parameters’
scores for all the products of the five queries, with
respect to the three considered databases.
3.3 Demonstration of Equivalence
In order to cover all the functions provided by
LEPRE, three medical devices emerged from the
analysis and were selected as references in the
demonstration of equivalence: device A, suitable for
the evaluation of the functions related to the lower
limb rehabilitation, device B, for the comparison of
the upper limb-related functions, and device C for the
evaluation of the cycloergometer-like features. The
main factors adopted to compare the devices’
characteristics, for both clinical and technical
evaluation, are collected in Table 4.
4 DISCUSSION
The analysis of the informative flow among
documents required by the normative framework
surely can provide the designer with a general
overview of requirements and potential constraints
for the device development. Indeed, the awareness of
the designer about this complex information net can
for instance anticipate potential criticalities in
technical solutions, like suggesting the exclusion of
not biocompatible materials where needed. This
scenario allows reducing resourcing, as time, costs
and human resources otherwise devoted to the
development of first attempt and not optimal
solutions (Amici et al., 2016). Nonetheless, given that
Table 3: Results and post-filtering documents with respect to included databases and selected search strings.
PubMed ClinicalTrials Cochrane
Keywords Search String
Found
results
Compatible
results
Found
results
Compatible
results
Found
results
Compatible
results
s1: neurological AND (robotic AND rehabilitation) 149 6 29 5 24 2
s2: orthopaedic AND (robotic AND rehabilitation) 29 2 0 0 10 1
s3: rehabilitation AND robot-assisted 118 5 58 4 16 0
s4: upper-limb AND (robotic AND rehabilitation) 176 2 55 4 19 0
s5: lower-limb AND (robotic AND rehabilitation) 76 4 7 2 14 0
Total 548 19 149 15 83 3
Design Approach of Medical Devices for Regulation Compatibility: A Robotic Rehabilitation Case Study
149
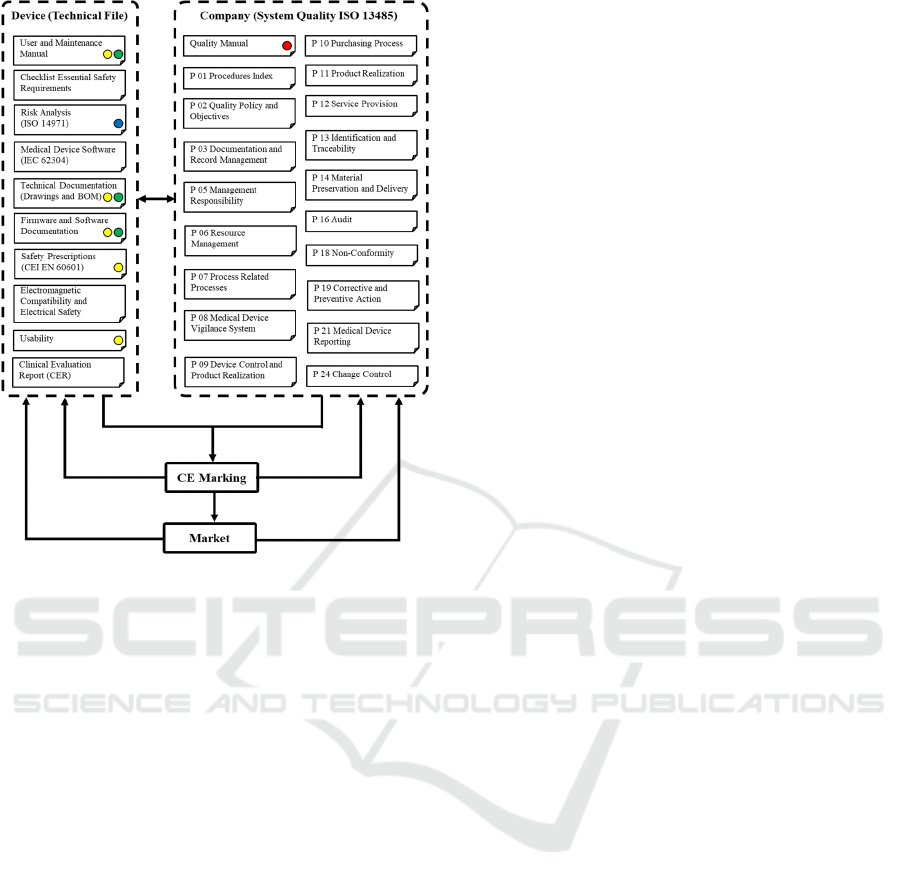
Figure 1: Schematization of information content and
mandatory documents required for the CE marking process.
The dashed boxes identify on the left the device-related
information expected within the technical file, and on the
right the manufacturer-related information required by the
System Quality ISO 13485 regulation. The yellow dots
indicate information referring to the description of the
LEPRE device, the green dots data related to the
demonstration of equivalence, finally the blue and red ones
depict risk analysis and post-market surveillance
information respectively.
those constraints derive from field-related regulatory
instruments, analogous analyses should be performed
when considering devices designed for different
operational environments, e.g. industrial applications,
according to a task-driven design strategy (Amici et
al., 2020), or more in general, a design-for-X
approach (Bause et al., 2019; Huang, 1996). In fact,
the scheme depicted in Figure 1 represents a custom
interpretation of the normative framework,
specifically developed within the scenario of medical
devices, designed for rehabilitative purposes, but also
provides an illustrative application of a generally
valid methodological approach. Besides, the need for
the same informative content among documents
suggests aiming for an optimization strategy also at a
process management level, in the documents’
definition. As a matter of fact, repeating the same
information in multiple instances should be a
deprecated strategy, since it leaves room for potential
mistakes or incongruences. Conversely, overall
knowledge of what information is required, for which
document, and with which level of detail, allows the
possibility of creating multi-purpose texts, suitable
for integration in different documents, with a modular
rationale.
Focusing on the illustrative case depicted in
Figure 1, LEPRE CER emphasizes strong first-level
connections with at least seven documents: for
instance, the device description is expected in User
and Maintenance Manual, Technical Documentation
(Drawings and Bill Of Materials – BOM), Firmware
and Software Documentation, Safety Prescriptions
(CEI EN 60601), as well as Usability document
within the documentation required by the device’s
technical file. In the same way, information related to
the demonstration of equivalence will be surely
included in the User and Maintenance Manual,
Technical Documentation (Drawings and BOM) and
Firmware and Software Documentation, although
differences apply also at this first approximation
level; for example, Firmware and Software
Documentation deals with both clinical and technical
equivalence, whereas the Technical Documentation
mainly focuses on the technical characteristics.
Besides, these interactions could be graphically
schematized as double arrowed connections between
two documents, since those relations should be
considered mutual. In the same way, schemes and
connections should be considered dynamic objects,
since they evolve with the design phase along the
development process. For instance, the introduction
of a new attachment able to provide new training
exercises would require an update of all the
documents device- and company-related, but P01
Procedure Index and P02 Quality Policy and
Objectives. Besides, also modifications at a software
level only, like the introduction of a new training
exercise which does not directly affect the device
hardware, would affect all the documents but
Technical Documentation (Drawings and BOM) in
addition to the previously cited P01 and P02. Still,
this result should not surprise, given that the data
interactions’ net reflects the complexity of the design
process (A.F. De Toni, 2007; A.F. De Toni and
Tonchia, 2002). Within this scenario, methodological
approaches aiming at the optimization of the design
process such as concurrent engineering solutions
could introduce considerable improvements in the
overall efficiency of the design process (Loureiro and
Curran, 2007).
Considering the CER developed for LEPRE, the
PubMed, ClinicalTrials and Cochrane databases have
been analyzed. Those databases present different
ICT4AWE 2021 - 7th International Conference on Information and Communication Technologies for Ageing Well and e-Health
150

characteristics and aims, and collect therefore
different kinds of data: the absence of duplicate
documents in the results of the corresponding search
strings among databases indicates that those
databases integrate each other, supporting the
appropriateness of analyzing them all. The analysis of
the clinical background emphasized the interest of
scientific research on this topic, especially in the last
years, as the ascending trend of the parameter p1
scores for the PubMed and ClinicalTrials databases
highlights. For the Cochrane database, a peak is
revealed for value 2; this behavior can be justified
considering that 2 points are assigned to a range of
years (2015-2017), unlike higher values, which refer
to single years. The high values of the p3 and p4
parameters support the suitability of the identified
search strings as detectors of the results’ clinical and
technical relevance respectively, since they allow
assessing population dimension, and importance and
applicability of the proposed scientific results.
Conversely, the p2 parameter can be considered a
valid indicator of methodological quality of the
investigation, since it expresses the correlation
between expected requirements imposed by the
search strings and actual content of the obtained
documents. A potential limitation of the proposed
approach is given by the evaluation of the ten most
recent documents for each query and each database,
but preliminary investigations suggested that this
value represented a reasonable compromise between
analysis quality and computational time. According
to this rationale, the number of evaluated documents
should be likely modified in case of analyses
performed on different fields, for instance, increased
when dealing with more traditional fields, like the
mechanical or the industrial one, which could
reasonably present a wider quantity of relevant
documents.
For the demonstration of equivalence, three
different devices had to be evaluated in order to
provide for a complete analysis of LEPRE device’s
functions, since no device currently on the market
provided a comparable set of features. The
comparison between LEPRE and device A allowed
demonstrating the clinical and technical equivalence
of the functions related to the mobilization of the
subject’s lower limb, since no differences
significantly affecting the equivalence can be
detected between the devices. In the same way, the
comparison with device B and device C allowed
fulfilling the clinical and technical equivalence for the
upper limb-related functions and the cycloergometer-
like features respectively. No demonstration of
biological equivalence was required since the user
shall wear gloves or socks while performing the
rehabilitative training with the LEPRE device. In fact,
in case of direct contact between patient and device,
MEDDEV 2.7/1 suggests the use of a biocompatible
material (International Organization for
Standardization, 2020) at a design process level, or
a)
b)
c)
d)
Figure 2: Bar plots of the assigned parameters’ scores in
aggregate form. From the top, the number of documents
with respect to the score and database, for a) publication
date (p1); b) accordance with search string (p2); c)
reference population (p3); and d) scientific consistency
(p4).
Design Approach of Medical Devices for Regulation Compatibility: A Robotic Rehabilitation Case Study
151

Table 4: Schematic comparison between LEPRE
characteristics and devices A, B and C respectively, with
respect to the parameters required for the clinical and
technical evaluation. Green checkmarks indicate a
complete overlapping of the characteristics between the
devices, whereas yellow checkmarks indicate a partial
equivalence: in the lower row of the table the detail of the
observed differences is presented.
A B C
Parameters for
Clinical Evaluation
Clinical condition
1
2
Intended purpose
Site of the body
involved
Reference
population
3
3
Parameters for
Technical Evaluation
Design structure
Conditions of use
Specifications and
properties
4
Deployment
methods
Principles of
operation
Differences Details
1. Device B can be adopted to also treat
pathologies related to the pelvic diaphragm
(e.g. incontinentia), currently excluded for
LEPRE device.
2. Device C can be adopted to also treat
pathologies such as hemodialysis, Alzheimer’s
disease/dementia,
percutaneous coronary
intervention (PCI), respiratory rehabilitation,
or poliomyelitis/post-poliomyelitis syndrome,
currently excluded for LEPRE device.
3. Reference population for the device also
includes pediatric subjects, currently excluded
for LEPRE device.
4. Device A presents overall dimensions and
weight remarkably higher than LEPRE device,
since it includes a seating system and supports
for the patient, unnecessary in LEPRE device.
the demonstration of biological equivalence at the
final stage. Nevertheless, the no-contact operational
condition assured by LEPRE also represents a
favorable asset for the use of rehabilitation devices
within the current scenario of the COVID-19
pandemic, easing the implementation of hygienizing
and sanitizing protocols.
5 CONCLUSIONS
The design process of medical devices must integrate
and optimize requirements related to technical and
clinical factors, user needs, therapy procedures, and
regulations’ constraints. The optimization of the
design process can be eased by the awareness about
the complex information net required by the
normative framework. Within this scenario, this
paper presents a design approach which aims at
easing the regulations compatibility of the designed
product, based on the custom mapping of required
data and referring documents for the development and
commercialization of a medical device according to
the CE marking process. This method is applied to the
illustrative case study of the LEPRE robotic system,
describing the data collection and analysis for the
device CER, as suggested MEDDEV 2.7/1
guidelines, with particular a focus on the evaluation
of clinical background and demonstration of
equivalence. Since the proposed method grounds on
the analysis of documentation that are strongly
dependent on the product operational conditions,
indications for the modifications required to adapt it
to further application fields are also suggested.
ACKNOWLEDGEMENTS
LEPRE was developed within the SIMeRiON project
(Innovative Mechatronics System for Orthopedic and
Neurological Rehabilitation), funded by Regione
Lombardia (bando FRIM FESR Aggregazioni
2016/18).
REFERENCES
Amici, C., Faglia, R., Taveggia, G., & Mor, M. (2016).
Development of User-Oriented Mechatronic Devices
for Post-Stroke Rehabilitation : the Experience of
UniBS H & W. Proceedings of R&D MANAGEMENT
Conf. 2016 from Science to Society: Innovation and
Value Creation 3-6 JULY 2016| CHURCHILL
COLLEGE | CAMBRIDGE Conf. Papers, (July), 1–10.
Amici, C., Ghidoni, M., Ceresoli, F., Gaffurini, P.,
Bissolotti, L., Mor, M., Fausti, D., Antonini, M., Ragni,
F., Tiboni, M. (2019). Preliminary Validation of a
Device for the Upper and Lower Limb Robotic
Rehabilitation. ICMT 2019 | 23rd International
Conference on Mechatronics Technology. Salerno.
Amici, C., Pellegrini, N., & Tiboni, M. (2020). The robot
selection problem for mini-parallel kinematic
machines: A task-driven approach to the selection
attributes identification. Micromachines, 11(8).
ICT4AWE 2021 - 7th International Conference on Information and Communication Technologies for Ageing Well and e-Health
152

https://doi.org/10.3390/MI11080711.
Bause, M., Khayamian Esfahani, B., Forbes, H., &
Schaefer, D. (2019). Design for Health 4.0: Exploration
of a New Area. Proceedings of the Design Society:
International Conference on Engineering Design, 1(1),
887–896. https://doi.org/10.1017/dsi.2019.93.
Ceresoli, F., Aggogeri, F., Amici, C., Borboni, A., Faglia,
R., Pellegrini, N., Tiboni, M., Antonini, M., Fausti, D.,
Mor, M., Petrogalli, G., Vertuan, A. (2019).
Differential system for gait rehabilitation.
MESROB2018, 1–8.
Clinical Trials. (n.d.). Clinical Trials. Retrieved from
https://www.clinicaltrials.gov/
Cochrane Library. (n.d.). Cochrane Library. Retrieved from
https://www.cochranelibrary.com/
Council of the European Union. (1993). Council Directive
93/42/EEC of 14 June 1993 concerning medical
devices. Official Journal of the European Communities.
Council of the European Union. (2007). Council Directive
2007/47/EC of the European Parliament and of the
Council of 5 September 2007 amending Council
Directive 90/385/EEC on the approximation of the laws
of the Member States relating to active implantable
medical devices, Council Directive 93.
De Toni, A. F., Fornasier, A., Montagner, M., & Nonino, F.
(2007). A performance measurement system for facility
management: The case study of a medical service
authority. International Journal of Productivity and
Performance Management, 56(5–6), 417–435.
https://doi.org/10.1108/17410400710757123.
De Toni, A. F., & Tonchia, S. (2002). New production
models: A strategic view. International Journal of
Production Research, 40(18), 4721–4741.
https://doi.org/10.1080/00207540210158005.
European Commission. (2016). Clinical Evaluation: a
Guide for Manufacturers and notified Bodies under
Directives 93/42/EEC and 90/385/EEC- 2.7/1 revision 4.
European Parliament; Council of the European Union.
(2017). Regulation (EU) 2017/745 of the European
Parliament and of the Council of 5 April 2017 on
medical devices, amending Directive 2001/83/EC,
Regulation (EC) No 178/2002 and Regulation (EC) No
1223/2009 and repealing Council Directives
90/385/EEC and 93/42/EE.
Fraser, A. G., Daubert, J. C., Van De Werf, F., Estes, N. A.
M., Smith, S. C., Krucoff, M. W., Vardas, P.
E.,Komajda, M. (2011). Clinical evaluation of
cardiovascular devices: Principles, problems, and
proposals for European regulatory reform. European
Heart Journal, 32(13), 1673–1686. https://doi.org/
10.1093/eurheartj/ehr171.
Hagedorn, T. J., Grosse, I. R., & Krishnamurty, S. (2015).
A concept ideation framework for medical device
design. Journal of Biomedical Informatics, 55, 218–
230. https://doi.org/10.1016/j.jbi.2015.04.010.
Henschke, C., Panteli, D., Perleth, M., & Busse, R. (2016).
Taxonomy of medical devices in the logic of health
technology assessment. International Journal of
Technology Assessment in Health Care,
31(5), 324–
330. https://doi.org/10.1017/S0266462315000562.
Huang, G. Q. (Ed.). (1996). Design for X.
https://doi.org/10.1007/978-94-011-3985-4.
International Organization for Standardization. (2016).
Medical devices — Quality management systems —
Requirements for regulatory purposes (ISO Standard
No. 13485:2016).
International Organization for Standardization. (2019).
Medical devices — Application of risk management to
medical devices (ISO Standard No. 14971:2019).
International Organization for Standardization. (2020).
Biological evaluation of medical devices — Part 18:
Chemical characterization of medical device materials
within a risk management process (ISO standard No.
10993-18:2020).
Kramer, D. B., Xu, S., Sc, M., & Kesselheim, A. S. (2020).
Regulation of Medical Devices in the United States and
European Union.
Loureiro, G., & Curran, R. (Eds.). (2007). Complex Systems
Concurrent Engineering. https://doi.org/10.1007/978-
1-84628-976-7.
National Center of Biotechnology Information. (n.d.).
PubMed. Retrieved from https://pubmed.ncbi
.nlm.nih.gov/
Privitera, M. B., Evans, M., & Southee, D. (2017). Human
factors in the design of medical devices – Approaches
to meeting international standards in the European
Union and USA. Applied Ergonomics, 59, 251–263.
https://doi.org/10.1016/j.apergo.2016.08.034.
Römer, G. W. R. B. E., & Stuyt, H. J. A. (2007). Compiling
a medical device file and a proposal for an international
standard for rehabilitation robots. 2007 IEEE 10th
International Conference on Rehabilitation Robotics,
ICORR’07, 00(c), 489–496. https://doi.org/10.1109/
ICORR.2007.4428471.
Schardt, C., Adams, M. B., Owens, T., Keitz, S., & Fontelo,
P. (2007). Utilization of the PICO framework to
improve searching PubMed for clinical questions. BMC
Medical Informatics and Decision Making, 7, 1–6.
https://doi.org/10.1186/1472-6947-7-16.
Design Approach of Medical Devices for Regulation Compatibility: A Robotic Rehabilitation Case Study
153
