
The Problem with ‘Dimensionless Quantities’
B. D. Hall
a
Measurement Standards Laboratory of New Zealand, New Zealand
Keywords:
SI, Quantities, Units, Dimensions.
Abstract:
Many different quantities can be described as ‘dimensionless’ and have the same SI unit “one”, so their different
natures cannot be determined from the unit name alone. In scientific unit systems like the SI, a set of base
quantities and associated units can lead to such many-to-one relationships between quantities, dimensions,
and units. So, it is necessary to understand how the mechanism of dimensions in unit systems works before
trying to design more general formats for the expression of quantities. In the familiar expression of physical
quantities, like mass, energy, etc, dimensions play a role when converting between unit systems or changing
unit prefixes. So, it is often assumed that the notion of dimension is general. However, this paper explains that
is not so: dimensions do not apply to all expressions of quantities. By recognising that there different types
of measurement scale, the role played by dimensions in systems like the SI can be seen as secondary to the
importance to the kind of quantity being expressed. There is a need to provide a unique way of identifying
each kind of quantity: dimensions and unit names are not able to fulfil this requirement.
1 INTRODUCTION
Digital support for physical quantities and units is
an elusive goal. In spite of efforts from as early as
the 1970s (Cleaveland, 1975; Gehani, 1977), noth-
ing seems to have found acceptance yet in quite the
same way as, say, numerical formats for representing
real numbers (e.g., the IEEE 754 Standard for Float-
ing Point Arithmetic). Perhaps one reason for slow
progress is that there is a wide variety of ways that
measurements can be expressed, reflecting the diver-
sity of measurement methods. There do not appear to
be clear guidelines on how to cater for different types
of measurement. The international metrology and sci-
entific communities have embraced the International
System of Units (SI) (BIPM, 2019), however, the SI
notation has, for pragmatic reasons, some shortcom-
ings (Foster, 2009; Mills, 2009).
The International Committee for Weights and
Measures (CIPM) has recognised the need to coor-
dinate digitalisation of the international measurement
system and established a Task Group on the Digital
SI (CIPM, 2019), supported by a team of experts. In
early 2021, this team made a public request for use-
cases to identify situations where digitalisation of ex-
isting metrological infrastructure might improve out-
comes or address difficulties. This paper presents a
a
https://orcid.org/0000-0002-4249-6863
preliminary analysis of one of those use-cases, which
identified redundant or ambiguous unit definitions as
a problem for interoperability.
1
For instance, if the
context in which units appear does not identify the
intended quantity, there are quite a few SI units that
are potentially ambiguous: heat capacity and entropy
(kg ⋅ m
2
⋅ s
−2
⋅ K
−1
), electric current and magnetomo-
tive force (A), torque and energy (kg ⋅ m
2
⋅ s
−2
), quan-
tities expressed in terms of inverse time (s
−1
), and
the gray and sievert (m
2
⋅ s
−2
) (BIPM, 2019, §2.3.4).
More generally, there is a large number of so-called
dimensionless quantities that all have the SI unit one.
The intent of this paper is to shed some light on the
internal mechanisms of unit systems like the SI. The
ambiguity attributed to some unit names arises from a
policy to compose the names for derived units from
the dimensional expression for the quantity and the
names of base units. The names produced this way are
helpful mnemonic devices when evaluating unit con-
version factors, however, unique names for each quan-
tity cannot be guaranteed. This is particularly obvious
in the case of dimensionless quantities.
By clarifying relationships between quantities, di-
mensions, and units, we show that support for a wider
range of expressions for measured quantities could be
1
The opinions expressed in this paper are those of the
author and are not necessarily shared by other members of
the CIPM Expert Group.
116
Hall, B.
The Problem with ‘Dimensionless Quantities’.
DOI: 10.5220/0010960300003119
In Proceedings of the 10th International Conference on Model-Driven Engineering and Software Development (MODELSWARD 2022), pages 116-125
ISBN: 978-989-758-550-0; ISSN: 2184-4348
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

developed. Dimensions and units play a role when
converting between different unit systems, but only
when quantities are expressed on a particular type
of measurement scale. The more general problem
of converting expressions between different types of
scale should be recognised. This leads us to suggest a
different way of organising support for quantities and
units.
2 QUANTITIES, DIMENSIONS,
UNITS, AND SCALES
In the 9
th
edition of the SI Brochure, we read that
(BIPM, 2019, §2.1)
The value of a quantity is generally expressed
as the product of a number and a unit. The unit
is simply a particular example of the quantity
concerned which is used as a reference, and
the number is the ratio of the value of the quan-
tity to the unit.
This is presumably an interpretation of the first para-
graph of the Preliminary chapter on measurement of
quantities in the Treatise on Electricity and Magnetism
(Maxwell, 1873)
Every expression of a Quantity consists of two
factors or components. One of these is the
name of a certain known quantity of the same
kind as the quantity to be expressed, which is
taken as a standard of reference. The other
component is the number of times the stan-
dard is taken in order to make up the required
quantity. The standard is technically called
the Unit, and the number is called the Numer-
ical Value of the quantity.
Maxwell’s description of units is an early instance of
metadata: a unit name (or symbol) is associated with
a value to allow the expression of a quantity to be in-
terpreted correctly in the context of different systems
of units. To see this, it is helpful to think in terms
of a producer and a consumer of data. A producer
expresses a quantity (a numerical value with a unit)
in such a way that consumers may interpret it cor-
rectly. Consumers ultimately need a numerical value
for computation. So, the producer’s expression of a
quantity, with a unit name, has to be reconciled with
the consumer’s measurement system. Maxwell de-
scribes this (Maxwell, 1873),
. .. a person of any nation, by substituting
for the different [unit] symbols the numerical
value of the quantities as measured by his own
national units, would arrive at a true result.
So, the unit name is a place-holder for the appropriate
numeric conversion factor (a numeric variable). This
factor is not known to the producer, because it depends
on the consumer context. Interoperability is enabled
by assigning the appropriate conversion factor in dif-
ferent measurement systems.
For instance, a minimum height requirement for
Police recruits may be expressed in imperial units as,
say, 5 f t 8 in. If this requirement is needed in a con-
text that uses SI units, appropriate values for unit sym-
bols are substituted: ft = 0.3048 and in = 0.0254 (the
values of one foot and one inch when measured in
metres). So, the minimum height in an SI context is
5 × 0.3048 + 8 × 0.0254 = 1.727.
It is perhaps important to emphasize that our con-
cern here is to communicate information about mea-
sured quantities. Maxwell was, of course, interested in
providing a concise mathematical formulation of the
physics of electromagnetism. By introducing the idea
of a unit system, he was able to describe relations be-
tween quantities without concern for units. This ab-
straction of quantities is now very familiar. For ex-
ample, a relation like Newton’s second law, 𝒇 = 𝒎𝒂,
is understood to be unit-independent; that is, the law
holds in any coherent unit system (the law is consid-
ered to hold even if system is not coherent, but one ad-
ditional numerical factor may be required). The terms
𝒇 , 𝒎, and 𝒂, in such equations are not simple numeric
variables; they are usually called quantities (bold italic
terms are used here for quantities).
Now, the relationship between an abstract unit-
independent quantity and a producer expression for
that quantity is of interest. Consider speed, which may
be defined without reference to units as 𝒗 = 𝒍∕𝒕, where
𝒕 is the time taken to cover a distance 𝒍. To express a
particular speed, a producer will assign a number to
{𝑣} and the name of the unit, or its symbol, to 𝑉 (e.g.,
mile-per-hour, knot, etc.). So, 𝒗 = {𝑣}𝑉 expresses a
speed in a general sense and 1.5 m ⋅ s
−1
is a particular
speed. The term 𝑉 is a place-holder for the unit name;
it is called the dimension of the quantity (Barenblatt,
1987). Valid substitutions for 𝑉 are names, or sym-
bols, for units for speed.
The general quantity expression for speed can be
related to expressions for length and time by substitut-
ing for each term in the defining equation,
𝒗 =
𝒍
𝒕
= {𝑣}𝑉
=
{𝑙}𝐿
{𝑡}𝑇
= {𝑙∕𝑡}𝐿𝑇
−1
.
Here terms in braces are values and dimensions are in
upper-case letters. The sense in which 𝐿 can be di-
vided by 𝑇 is that numbers (conversion factors) are
ultimately assigned to the unit names substituted for
The Problem with ‘Dimensionless Quantities’
117

these dimensions. When the expression of a quantity
is reduced to a number in a particular unit system, the
unit terms act as conversion factors. This is quite im-
portant: when units for length and time change, an ex-
pression like 𝐿𝑇
−1
describes the scaling required for
a speed (the factor for speed is equal to the conversion
factor for the unit of length divided by the conversion
factor for the unit of time).
Starting with a small independent basis set of
quantities (mass, length, and time), and using (unit-
independent) equations of physics, Maxwell defined
other quantities of interest. This simultaneously es-
tablished relationships between the units chosen to ex-
press those quantities. The resulting structure is useful
when converting between unit systems, because con-
version factors can be derived from the base units. To
this end, derived unit names often encode a quantity’s
dependence on base quantities. For example, the name
of the SI unit for acceleration is “metre-per-second-
per-second” (m ⋅ s
−2
), which is obtained by substitut-
ing ‘metre’ and ‘second’ into the dimensional expres-
sion 𝐿𝑇
−2
.
Because unit names are derived from dimensional
expressions, they encode the scaling relationships but
the nature of the derived quantity is not necessarily
captured. The SI Brochure concedes that . .. several
different quantities may share the same SI unit (BIPM,
2019, §2.3.4). The most obvious examples are the
many different kinds of dimensionless quantities that
all have the SI unit one. This unit indicates that no
scaling is needed when base units change (e.g., an as-
pect ratio does not depend on units of length), but the
unit name gives no hint as to the nature of the quan-
tity itself. There are also dimensioned quantities in the
SI that are indistinguishable, such as torque and work
which have the dimension 𝑀 𝐿
2
𝑇
−2
. The SI includes
some special unit names to try to mitigate this prob-
lem. For instance, plane angle may be expressed in
rad or in m ⋅ m
−1
, activity in Bq or in s
−1
, etc. Nev-
ertheless, the composed names for these units are also
considered valid.
The construction of a system of quantities must
assume that the multiplication and division of terms
in defining equations is meaningful (like 𝒗 = 𝒍∕𝒕 and
𝒇 = 𝒎𝒂). However, that places limits on the type of
measurement scale that can be used to express terms:
only ratio scales (Stevens, 1946), which are charac-
terised by a scale-independent zero, are acceptable
(e.g., the foot, inch, furlong and metre are all ratio
scales for length; on each, zero corresponds to the
same length).
However, there are other types of scale in use,
which should be considered. Stevens identified four
categories of scale and Chrisman added several more
levels (Chrisman, 1995; Chrisman, 1998). The scales
identified by Stevens can be classified by the mathe-
matical transformations that leave a scale’s form in-
variant; the four scales are called: nominal, ordinal,
interval and ratio.
For instance, if 𝑥 and 𝑥
′
are values for the same
quantity expressed on different ratio scales, there will
be some conversion factor 𝐾 such that 𝑥
′
= 𝐾 𝑥, which
preserves the form of the scale. For interval scales,
an affine transformation of the form 𝑥
′
= 𝐾 𝑥 + 𝑂 is
needed (c.f., conversion between Fahrenheit and Cel-
sius); for ordinal scales, a monotonic increasing func-
tion 𝑥
′
= 𝑓 (𝑥) is required. We may think of these
different forms of transformation as generalisations of
the simple conversion factors that convert between ra-
tio scales for things like mass and length.
If different types of scale are an important classi-
fier, the term ‘quantity’ must be given some consid-
eration. In the most general sense, a quantity may
be thought of as an attribute of some phenomenon,
body or substance, that can be distinguished quali-
tatively and determined quantitatively (BIPM et al.,
1984, §1.01). Such a quantity might be expressed on
any of Stevens’s scales (e.g., a body can be determined
to have a temperature, that temperature can be mea-
sured on an ordinal scale by a thermochromic device,
or on an interval scale by a domestic thermometer, or
on a ratio scale by a radiation thermometer). How-
ever, this interpretation of the term quantity may be
too broad for our purposes and could lead to confu-
sion. As already noted, the terms in unit-independent
equations—which are often referred to as quantities—
are not quantities in this general sense.
To a physicist or applied mathematician, it is al-
most universally assumed, although rarely acknowl-
edged, that ‘quantity’ is used in the restricted sense
that applies to unit-independent expressions. The
terms appearing in equations such as Newton’s law
stand for a quantity that can be expressed on a ratio
scale. A great deal of published material on quanti-
ties, units, and dimensions is written from this point
of view. Because of this, we believe that ‘quantity’
must be used in this restricted sense, and be quali-
fied appropriately when a different notion is intended
(such as, ‘ordinal quantity’ or ‘interval quantity’; to
indicate the more general sense ‘general quantity’ or
‘kind of quantity’ may be used ). This means that all
quantities have an associated dimension, and perhaps
a dimensional expression related to the quantity def-
inition. Conversely, the notion of dimension is only
meaningful for quantities in the stricter sense; it does
not apply to any other types of measurement scale, be-
cause simple scaling does not leave those scales in-
variant. This limitation of dimensions is often misun-
MODELSWARD 2022 - 10th International Conference on Model-Driven Engineering and Software Development
118
derstood.
Indeed there appears to be little awareness about
the different types of measurement scale. A recent re-
view, by Grozier, found that the distinction between
unit dependence and independence is often unclear.
He observed “. . . unit-invariance does not appear to
have yet entered the scientific mainstream; instead,
formulae which are unit-invariant are often not recog-
nised as such, and formulae that are unit-dependent
are often wrongly taken to be of universal application”
(Grozier, 2020). This observation is somewhat alarm-
ing and suggests that, in striving for more autonomous
digital systems, we must apply stricter discipline when
describing physical data and metadata.
2.1 Quantity and Scales
The qualified terms ‘nominal quantity’, ‘ordinal quan-
tity’, or ‘interval quantity’ may be used to refer to
quantities expressed on corresponding types of scale.
The arithmetic, ordering and equivalence operations
that apply to data expressed on the different scales can
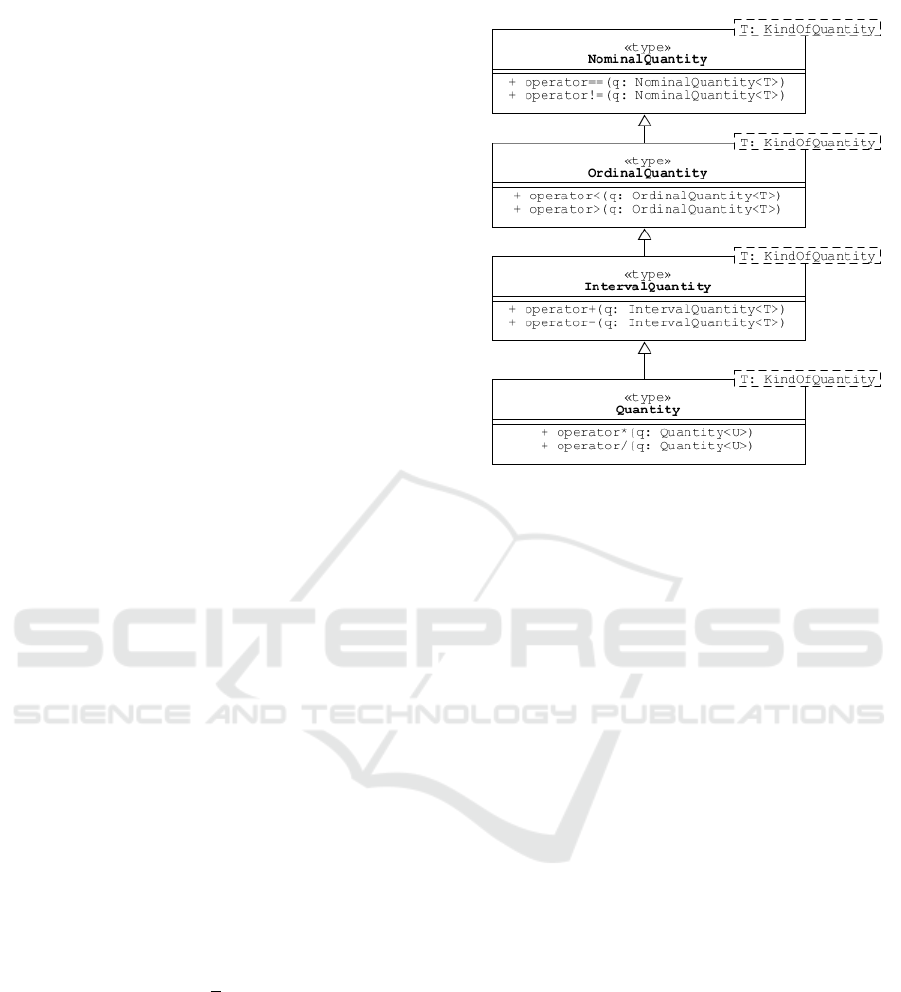
be organised as shown in the class diagram in Fig-
ure 1. The base class corresponds to a nominal quan-
tity; one can only determine whether an object pos-
sesses a particular nominal quantity or not. However,
an ordinal quantity has a magnitude that can be mean-
ingfully compared; although, it is not meaningful to
compare the differences between pairs of magnitudes.
For an interval quantity, the difference between mag-
nitudes is meaningful but not ratios of the magnitudes
(e.g., if the air temperature yesterday was 15 °C and
today it is 20 °C, it makes sense to say that it is 5 °C
warmer today, but not that it is 33 % warmer). Finally,
because ratio scales have a natural zero (e.g., zero feet
is the same as zero metres, etc), ratios of magnitudes
are meaningful.
In terms of software, distinguishing between the
different levels of expression could exert tighter con-
trol over declarations and assignments to variables.
For instance, the physics equation
𝑬 =
3
2
𝒌
B
𝑻 (1)
is unit-independent; 𝑬, 𝒌
B
and 𝑻 represent quantities:
𝑬 is the average translational kinetic energy of atoms
in an ideal gas, 𝒌
B
is a proportionality constant be-
tween energy and temperature, called the Boltzmann
constant, and 𝑻 is thermodynamic temperature. Now,
the relationship between quantities on either side of
this equation is constrained and so the types chosen to
represent terms can be validated. If the result of the
expression is declared an energy, and a term associ-
ated with 𝑻 is declared a temperature, then the term
representing the Boltzmann constant must be declared
Figure 1: This UML class diagram shows a simple inher-
itance relationship between four different levels of expres-
sion for a general quantity. Stevens identified nominal, or-
dinal, interval and ratio scales as different levels of expres-
sion (Stevens, 1946). It is useful to formalise these levels
(and perhaps others (Chrisman, 1995; Chrisman, 1998)), in
terms of the operations that are meaningful for data. Use
of the term ‘quantity’ here, for ratio scales, corresponds to
common usage in physical science; the more general sense
of quantity is represented by the template parameter for a
particular kind of quantity. Note the product and quotient
operations defined for a Quantity<T> may take a differ-
ent quantity kind argument Quantity<U>. These operations
generally return a different quantity kind too (not shown).
a quantity that relates temperature to energy. This con-
sistency check could be regarded as application of the
principle of dimensional homogeneity, which requires
that the dimensions of each term in a unit-independent
equation be the same. However, ambiguity between
quantities can still arise if the homogeneity principle
is applied using a fixed set of base dimensions, such
as in the SI. To avoid this, some form of unambiguous
representation of the quantities themselves is required
and, if quantity expressions are to be checked, some
way of representing relationships among quantities is
also needed. How to provide this information is an
open question, which will be discussed further below.
Let us suppose that appropriate quantity-specific
terms have been declared to represent equation (1).
The computation itself can only be carried out when
quantities are expressed on appropriate an appropriate
choice of ratio scales (for instance, in SI units). How-
ever, if the data assigned to terms are not immediately
in the correct form, a digital system could apply cer-
tain pre- or post-processing steps automatically. For
The Problem with ‘Dimensionless Quantities’
119

instance:
1. Appropriate conversion factors could be applied,
if the assigned data were expressed as a quan-
tity, but not in the units required. For example,
there is an Imperial scale for absolute tempera-
ture called Rankine (symbol
◦
R). If a value for
𝑻 were expressed in
◦
R, the conversion factor
273.15∕491.67 = 0.555 56 K∕
◦
R could be applied
to obtain an expression in kelvin.
2. Conversion of interval-quantity data to a suitable
quantity scale could be carried out. For exam-
ple, an absolute temperature expressed in degrees
Fahrenheit could be converted to kelvin by an
affine transform. This would also coerce the data
type from IntervalQuantity<Temperature> to
Quantity<Temperature>, but the legitimacy of
this operation could be determined by the system,
because the kind of quantity—temperature—is the
same.
3. The format for reporting results could be adapted
to user requirements. For example, quantities that
are subject to formal regulations generally require
metrological traceability to SI units. Nevertheless,
reporting in customary units may be desirable re-
quiring conversion of results to a different form.
Such conversion might also involve type-coercion
to interval or ordinal quantities.
4. If a particular assignment cannot be made le-
gitimately, an exception could be raised to pro-
vide an opportunity for further ad hoc pro-
cessing. For example, if temperature data
were expressed as an ordinal quantity (i.e.,
as an OrdinalQuantity<Temperature>), auto-
matic conversion to a quantity is illegal. Neverthe-
less, information about the quantity could perhaps
be handled by an exception. For instance, an upper
or lower bound on temperature might be known,
so a range of corresponding results could be eval-
uated.
3 THE PROBLEM WITH
‘DIMENSIONLESS
QUANTITIES’
The title of this paper is intended to draw attention
to the predominance of base dimensions as a proxy
for quantities in our thinking and, indeed, to how mis-
leading that is. Introducing the notion of scales (lev-
els of measurement) has shown that the notion of a
dimension applies only to quantities expressed on a
ratio scale. There are more general aspects that must
be captured. Indeed, were it not for the overwhelm-
ing use of ‘quantity’ in scientific literature, we would
have suggested that ‘dimension’ could be adopted as
a name for the (ratio) quantity type. Unfortunately,
‘quantity’ and ‘dimension’ have become hopelessly
entangled (Emerson, 2005).
So, if there is a problem with dimensionless quan-
tities, it appears to be a semantic one. A physicist
would consider quantities, such as the gain of an am-
plifier, a refractive index, or an emissivity, to tran-
scend measurement; a property would be assumed to
exist independently of any consideration about how
to measure it. Yet, a physicist would also take for
granted that mathematical models—unit-independent
equations—could incorporate terms for quantities like
amplifier gain, refractive index, etc, which are dimen-
sionless. This restricts the expression of those terms
to ratio scales (there could be no such thing as a ‘di-
mensionless interval quantity’, for example).
The fact that a particular unit can be used to ex-
press several different quantities is not a problem. The
unit one is appropriate for all dimensionless quantities,
because it indicates to the consumer that the value in
the producer expression does not require conversion.
Nonetheless, the expression of a dimensionless quan-
tity is not a pure number: it is merely insensitive to
changes in the units of the measurement system. The
unit one arises from arithmetic cancellation of dimen-
sion variables (factors, or equivalently unit names).
For example, an aspect ratio is the ratio of two lengths
𝒂 =
𝒍
1
𝒍
2
Using the full expression of quantities
{𝑎}𝐴 =
{𝑙
1
}𝐿
{𝑙
2
}𝐿
=
{𝑙
1
}
{𝑙
2
}
,
because the factor 𝐿∕𝐿 reduces to unity for any choice
of unit and so 𝐴 = 1.
Dimensionless quantities arise when there is com-
plete cancellation of dimension variables or unit
names, however partial cancellation can occur as well.
Perhaps the best-known example is that of work and
torque (both having the derived SI unit kg ⋅ m
2
⋅ s
−2
).
These quantities would have different dimensions, and
therefore different names, if angle were included as a
base quantity in the system (Brownstein, 1997). How-
ever, angle is defined as a ratio of lengths in the SI and
so its role in the definition of torque escapes from the
dimensional representation. Other examples of ambi-
guity were given in the Introduction. Again, this is
not the symptom of a problem: there should not be
MODELSWARD 2022 - 10th International Conference on Model-Driven Engineering and Software Development
120

an expectation that a unit name will uniquely iden-
tify a quantity. Dimensional expressions will always
correctly represent the conversion factors needed to
change units, but that is all.
4 ORGANISING INFORMATION
The more general notion of quantity is a common
thread among the different forms of expression for
quantities. For example, temperature may be ex-
pressed in kelvin, in degrees Celsius, in degrees
Fahrenheit, or in comparison to some particular ref-
erence temperature, and we may legitimately convert
between different forms of expression as long as the
kind of quantity—temperature—remains common to
each. So, to represent quantities and units of measure-
ment in digital systems, the kind of quantity should be
given prominence. Furthermore, to enable interoper-
ability among systems, identifiers for different kinds
of quantity, as well as units of measurement, should
be standardised.
The task of developing detailed digital registers
containing information about the huge number of
common units and even greater number of quantity
kinds is daunting. Nevertheless, a number of ini-
tiatives do provide references for units of measure.
These projects have established substantial user com-
munities and are well maintained. All, however, have
adopted internal structures based on the Maxwellian
model for unit systems. We briefly present three of
these projects.
UCUM: The Unified Code for Units of Measure
(UCUM) in clinical information systems claims to
provide a complete and unambiguous coding sys-
tem for units.
"The Unified Code for Units of Measure is a code
system intended to include all units of measures
being contemporarily used in international sci-
ence, engineering, and business. The purpose is to
facilitate unambiguous electronic communication
of quantities together with their units (Schadow
et al., 1999)."
UCUM is designed around the mathematical prop-
erties of a fixed basis of units. Unit are defined by a
numeric factor and a sequence of base-unit expo-
nents. Units for interval quantities are treated as
exceptions (“special units”) and there is also the
notion of “arbitrary unit”, which bears no relation
to any other unit. A unit may also be adorned with
a textual “annotation”, although this does not con-
tribute to the unit semantics.
OM: The Ontology of units of Measure (OM) pro-
vides a conceptual framework for units of mea-
surement (Rijgersberg et al., 2013). It implements
classes, instances, and properties intended to be
used to represent measurement concepts and in-
cludes various systems of units.
The notion of quantity corresponds to the narrow
sense of the term. However, OM distinguishes be-
tween a ‘measure’ and a ‘scale’, the former cor-
responding to the expression of a quantity and
the latter to the expression of an interval quan-
tity. This allows OM to use the degree Celsius and
the degree Fahrenheit in two quite different ways
(given in the OM documentation as an example).
Temperature can be expressed on an ‘scale’, which
allows an offset origin, so 0 °C ≠ 0 °F ≠ 0 K, or, as
a ‘measure’, it is admissible to write 0 °C = 0 °F =
0K, where the expressions represent quantities (in
this case, temperature differences).
QUDT: The Quantity Units Dimensions and Types
(QUDT) ontology is intended to model quantities,
units, numerical values, and data types to store and
manipulate these objects (QUDT, 2021).
QUDT’s semantics are constructed on a basis set
of quantities and units and it claims to establish a
relationship between units and quantity kinds by
the internal representation of dimensions. This
claim will be limited, because a dimensional rep-
resentation cannot uniquely identify quantities.
However, to overcome some of the problems asso-
ciated with dimensionless quantities, QUDT uses
a numerator and denominator of dimensional ex-
ponents in the internal representation.
The interest in ontologies is intended to capture con-
ceptual information (knowledge) to associate with em-
pirical data stored in digital records. If this is done
consistently on a large scale it may enable applica-
tions of artificial intelligence (AI) and machine learn-
ing (ML) on large heterogeneous collections of phys-
ical data.
The fact that ontologies have so far focused on the
narrow meaning of quantity may not be an immediate
problem, if applications of AI and ML use scientific
data. However, poor representation of metrological
concepts in knowledge discovery tools is ultimately of
concern. Vast quantities of physical data will be gen-
erated by digital sensor networks and much of this data
will not be expressible as quantities. How is this data
to be represented in a way that autonomous machines
could make use of it?
We must recognise that the general notion of quan-
tity is fundamental and distinct from units and also
that units may be used to express more than just
simple ratio quantities. It is necessary to uniquely
identify kinds of quantity independently of units.
The Problem with ‘Dimensionless Quantities’
121

The full expression of a quantity has to include: a
value, a unit and a quantity kind, as indicated by the
QuantityExpression class shown in Figure 2.
Figure 2: The digital expression of a quantity will be com-
posed of: a value, a unit, and a quantity kind. This would
be applicable to all of the types shown in Figure 1 (a ‘mixin’
class). The properties of different kinds of quantity would be
handled by applications, so only a unique identifier is shown.
An elegant feature of Maxwellian unit systems is
that a producer of data need not anticipate consumers’
requirements: consumers are responsible for conver-
sions from the producer’s unit. So, a digital unit must
also carry enough information to allow conversion. A
simple schema for digital units is shown in Figure 3. A
unit must uniquely identify the combination of: a unit
name, a unit system and a type of scale. For example,
degrees Celsius in the SI system would have two dig-
ital units: one for temperature expressed as an inter-
val quantity, the other for temperature as a ratio quan-
tity. These units would avoid an existing source of
ambiguity, where a consumer does not know whether
a temperature expressed in degrees Celsius is offset.
A temperature difference expressed in degrees Cel-
sius would use the digital unit for a quantity, whereas,
an absolute temperature expressed in degrees Celsius
would use the unit for an interval quantity.
Figure 3: A digital unit carries information about the context
of an expressed quantity to allow conversion to an equivalent
form in a different context. The identifier must be unique to
the combination of the name, the unit system (e.g., SI, CGS,
etc), and the type of scale (ratio, interval, etc).
To convert between different expressions of a
quantity may involve more than just a multiplicative
factor. Conversion functions depend on the types of
scale involved and hence on the producer (source) and
consumer (destination) units, as indicated in Figure 4.
Figure 4: Unit conversion will apply an operation to the nu-
meric value of an expressed quantity. The appropriate con-
version function depends on both the source (producer) and
destination (consumer) units (src_unit and dst_unit).
An authoritative register of quantities and ex-
tended unit representations would be convenient to
support the expression of quantities and units. En-
during unique identifiers are needed for both quantity
kinds and units, and a central reference of unit con-
version operations is also desirable. Figure 5 shows
the idea. Of course, such a repository would be likely
to curate more extensive information about the quan-
tities, units and unit systems, as is the case for UCUM,
OM, and CUDT.
5 DIMENSIONLESS QUANTITY
CALCULUS
Inferring the type of quantity that results from an ar-
bitrary computation has often been a motivation for
looking at digital representations for quantities and
units (e.g., (Kennedy, 2010)). However, that is a dif-
ferent problem to developing suitable formats for the
full expression of quantities.
The notion of quantity used in calculations is lim-
ited to expressions on ratio scales and only fairly ba-
sic operations are permissible (essentially just arith-
metic). Type-inference for such calculations must ex-
ploit a notion of quantity that is independent of base
units and dimensions. The idea of using the base di-
mensions of a unit system to generate an abstract lat-
tice of distinct quantities fails to distinguish between
all possibilities (De Boer, 1995). However, by sep-
arating unit representations from quantities, progress
can be made. Recent work has shown that many dif-
ficult cases can be handled by declaring quantities of
importance in context (Hall, 2020).
Dimensionless quantities are considered a special
case in computations. They are often regarded as pure
numbers. However, this point of view should not be
adopted for digitalisation of quantity calculus. Di-
mensionless quantities are indeed different from di-
mensioned quantities, but only by virtue of their dif-
ferent scaling behaviour when units change.
To see this more clearly, consider a length 𝒍. The
dimension of 𝒍 is 𝐿 and the dimension of 𝒍
2
is 𝐿
2
.
So, the sum 𝒍 +𝒍
2
cannot be expressed as a quantity in
the form 𝒒 = {𝑞}𝑄; that is why this operation is not
MODELSWARD 2022 - 10th International Conference on Model-Driven Engineering and Software Development
122

Figure 5: An authoritative register of information would be needed to support interoperability of digital representations for
quantities and units. Enduring and unique identifiers are needed for both quantity kinds and (digital) units, and a central
reference for operations to convert between different units is also desirable.
permitted. On the other hand, for a dimensionless as-
pect ratio 𝒂, we may indeed express the sum of powers
𝒂 + 𝒂
2
+ ⋯ as a quantity (each term is dimensionless).
For this reason it is meaningful, and therefore permis-
sible, to apply more general mathematical functions
to the value of a dimensionless quantity. A different
quantity will generally result, but both will be dimen-
sionless. For example, the trigonometric sine function
transforms an angle (angle is a dimensionless SI quan-
tity and the sine function can be expressed as Taylor
series) into a quantity that may be interpreted as the
ratio of perpendicular sides in a right triangle.
6 DISCUSSION
The SI provides a framework for effective communi-
cation of information about physical quantities, but the
fundamental assumption, that . . . a quantity is gener-
ally expressed as the produce of a number and a unit
is too limited. A third aspect is needed: the quantity
must be identified. It is the absence of this additional
information that makes dimensionless quantities hard
to handle.
If we accept that each kind of quantity must be
identified independently of the unit in which it is ex-
pressed, then the stage is set for much informative
quantity expressions. The different levels of measure-
ment (scales) that are used in society can be handled
directly, not as exceptions, and the semantics associ-
ated with expressed values can be properly captured.
One of the arguments for adopting SI units over
conventional units is that conversion between units
would not be needed if everyone used the SI. How-
ever, conventional units have proved very resistant to
change and the ubiquity of digital systems now greatly
facilitates conversions. Moreover, SI units do not ap-
ply to most non-ratio scale expressions.
An important class of such measurements are com-
monly reported in SI units although they cannot be
expressed on ratio scales. Many measurements of ma-
terial properties fall into this category (Kirkham and
White, 2018). Such measurements may follow a stan-
dardised procedure that renders values for a particular
purpose. However, when measured by different proce-
dures, the same nominal property may give quite dif-
ferent results. The characterisation of viscosity is one
example where there are literally dozens of methods
(for details, see (Kirkham and White, 2018)). In such
cases, expressed quantities could be better handled by
identifying the method used with the quantity kind.
This would prevent misinterpretation.
In 2019, the SI underwent a very significant
change when exact values for seven ‘defining con-
stants’ were adopted as a way of constructing the sys-
tem (BIPM, 2019). This finally retired ‘base units’, al-
though they have been retained to support legacy ver-
sions of the SI and the SI notation. Emphasis is now
placed on the equations of physics that establish re-
lationships among quantities. So, although the unit
of speed remains the ‘metre-per-second’, we might
equally think of it as ‘SI-speed’; there would be no
harm in doing so. The change to the SI adds weight to
our suggestion that each digital unit deserves an indi-
vidual unique identifier. There is no hierarchy among
units to be captured in the representation.
It is not possible to attribute a cost due to in-
correct interpretations of physical data. A few well-
known stories, like the NASA Mars Climate Ob-
server (Oberg, 1999), or the Gimli Glider incident
(Wikipedia, 2021a), are often cited to motivate the
need for better handling of units. The huge disruption
caused by the practice of storing a year as two dec-
imal digits (the Year 2000 problem) has been better
documented (Wikipedia, 2021b). Another, less well-
known, story involves the construction of a bridge
over the Rhine river, where there was confusion about
height expressed on interval scales (BBC, 2014). The
so-called ‘height above sea level’ is an interval scale
for length, because the origin—sea level—is arbitrar-
The Problem with ‘Dimensionless Quantities’
123

ily defined. In Germany, sea level is taken with re-
spect to the North Sea, whereas Switzerland refers to
the Mediterranean Sea. These heights are not equiv-
alent, there is a nominal difference of 27 cm between
them. So, when a bridge was built between Germany
and Switzerland, at Laufenburg, the difference be-
tween expressions of height needed to be accounted
for, but was mishandled, resulting in a 54 cm height
difference between the Swiss and German sides. Ev-
eryone would agree, it seems, that more reliable ways
of communicating information about physical quanti-
ties are needed, as well as a greater awareness of the
metrological concepts involved.
7 CONCLUSIONS
In summary, the problem with dimensionless quanti-
ties, and more generally with dimensions themselves,
is that often it is incorrectly assumed that a dimension
or a unit can identify a corresponding quantity. We
have explained how the manipulation of conventional
units, as explained by Maxwell, can lead to such am-
biguity. This is not a problem with the units, rather
it is a misinterpretation of their role. Several obser-
vations can be made: dimensional expressions, and
compound unit names based on such expressions, can-
not reliably identify quantities; however, dimensional
expressions can always be relied upon to obtain con-
version factors when units change.
The mathematical modelling of a system of quan-
tities, like the SI, assumes that quantities are expressed
on ratio scales. However, other types of scale are
important too. To accommodate a wider range of
measurements, quantity expressions should be gener-
alised. The various levels of measurement proposed
by Stevens and Chrisman offer a useful classification
of scales and can be used to implement wider sup-
port for physical data. The different levels can be rep-
resented by simple hierarchical inheritance structures
(like Figure 1).
The term ‘quantity’ must be handled carefully.
The common use of quantity by physical scientists
should be adopted for expressions on ratio scales
and the more general notion of quantity—as a prop-
erty that exists without consideration for a particular
form of expression—should be suitably qualified. The
terms ‘nominal quantity’, ‘ordinal quantity’, and ‘in-
terval quantity’ could be adopted to refer to expres-
sions on nominal, ordinal, or intervals scales and other
terms may be introduced if the levels proposed by
Chrisman are adopted.
The more general notion of quantity is fundamen-
tally important. Conversion between different expres-
sions of the same quantity may be possible, up or
down a hierarchy of scales (e.g., a body might be
deemed to have a temperature (nominal), that temper-
ature could be ranked against other temperatures (or-
dinal), or given a value in degrees Celsius (interval),
or kelvin (ratio)). For this reason, Figure 1 used the
parameter T to represent the kind of quantity. A digi-
tal system should adopt suitable unique identifiers for
each general quantity. Having these identifiers avail-
able would immediately resolve the difficulties that
prompted the preparation of this report.
ACKNOWLEDGEMENTS
The author is grateful to Vladimir Bubanja and Joe
Borbely for careful review of this manuscript. This
work was funded by the New Zealand government.
REFERENCES
Barenblatt, G. I. (1987). Dimensional analysis. Gordon and
Breech.
BBC (2014). The Laufenburg bridge. https://www.bbc.
co.uk/news/magazine-27509559 (accessed 28 Dec.
2021).
BIPM (2019). The International System of Units. BIPM,
Sèvres, France, 9
th
edition.
BIPM, ISO, IEC, and OIML (1984). Vocabulary of metrol-
ogy, Part 1, basic and general terms (international).
International Organisation for Standardization.
Brownstein, K. (1997). Angles—let’s treat them squarely.
American Journal of Physics, 65(7):605–614.
Chrisman, N. R. (1995). Beyond stevens: A revised ap-
proach to measurement for geographic information. In
AUTOCARTO-CONFERENCE, pages 271–280.
Chrisman, N. R. (1998). Rethinking levels of measurement
for cartography. Cartography and Geographic Infor-
mation Systems, 25(4):231–242.
CIPM (2019). CIPM Task Group on the Digital SI. (ac-
cessed 29 August 2021). https://www.bipm.org/en/
committees/ci/cipm/wg/cipm-tg-dsi.
Cleaveland, J. C. (1975). Pouches, a programming language
construct encouraging redundancy. Msc, University of
California, Los Angeles–Computer Science.
De Boer, J. (1995). On the history of quantity calculus and
the international system. Metrologia, 31(6):405.
Emerson, W. H. (2005). On the concept of dimension.
Metrologia, 42(4):L21–L22.
Foster, M. P. (2009). Disambiguating the SI notation would
guarantee its correct parsing. Proceedings of the Royal
Society A: Mathematical, Physical and Engineering
Sciences, 465(2108):1227–1229.
Gehani, N. (1977). Units of measure as a data attribute.
Computer Languages, 2(3):93–111.
MODELSWARD 2022 - 10th International Conference on Model-Driven Engineering and Software Development
124

Grozier, J. (2020). Should physical laws be unit-invariant?
Studies in History and Philosophy of Science Part A,
80:9–18.
Hall, B. D. (2020). Software for calculation with physi-
cal quantities. In 2020 IEEE International Workshop
on Metrology for Industry 4.0 & IoT, pages 458–463.
IEEE.
Kennedy, A. (2010). Types for Units-of-Measure: Theory
and Practice. In Horváth, Z., Plasmeijer, R., and Zsók,
V., editors, Central European Functional Program-
ming School, volume 6299, pages 268–305. Springer
Berlin Heidelberg, Berlin, Heidelberg. Series Title:
Lecture Notes in Computer Science.
Kirkham, H. and White, R. (2018). Pure and applied mea-
surement: The need for expanded education. IEEE
Instrumentation & Measurement Magazine, 21(6):52–
59.
Maxwell, J. C. (1873). A Treatise on Electricity and Mag-
netism. Clarendon Press, Oxford.
Mills, I. (2009). Conventions for writing the values of
quantities in the SI. Proceedings of the Royal Society
A: Mathematical, Physical and Engineering Sciences,
465(2108):2313–2316.
Oberg, J. (1999). Why the Mars probe went off course. IEEE
Spectrum, December:34–39.
QUDT (2021). Quantity, Unit, Dimension and Type. (ac-
cessed 26 March 2021). DOI: 10.25504/FAIRshar-
ing.d3pqw7.
Rijgersberg, H., van Assem, M., and Top, J. (2013). Ontol-
ogy of units of measure and related concepts. Semantic
Web, 4(1):3–13. https://github.com/HajoRijgersberg/
OM.
Schadow, G., McDonald, C. J., Suico, J. G., Föhring, U.,
and Tolxdorff, T. (1999). Units of measure in clinical
information systems. Journal of the American Medical
Informatics Association, 6(2):151–162. https://ucum.
nlm.nih.gov/.
Stevens, S. S. (1946). On the theory of scales. Science,
103(2684):677 – 680.
Wikipedia (2021a). Gimli Glider. https://en.wikipedia.org/
wiki/Gimli_Glider (accessed 28 Dec. 2021).
Wikipedia (2021b). Year 2000 problem. https://en.
wikipedia.org/wiki/Year_2000_problem (accessed 28
Dec. 2021).
The Problem with ‘Dimensionless Quantities’
125
