
Study on Respiratory Metabolism of Wild Acanthopagrus schlegelii in
South China Sea under Different Temperatures and Weights
Xue Feng
1a
, Jiangtao Fan
1b
, Xueqian Zhao
2c
and Pimao Chen
1d
1
South China Sea Fisheries Research Institute, CAFS/ Scientific Observing and Experimental Station of South China Sea
Fishery Resources and Environment, Ministry of Agriculture and Rural Affairs, P.R. China/ Guangdong Engineering
Technology Research Center of Marine Recreational Fishery, Guangzhou, Guangdong, 510300, China
2
Jinan Zoo, Jinan, Shandong, 250031, China
Keywords: Acanthopagrus schlegelii, Oxygen Consumption Rate, Ammonia Excretion Rate, Water Temperature,
Weight.
Abstract: Respiratory metabolism is an important part of bioenergy research. The experimental device of indoor closed
flowing respiratory metabolism designed and authorized under an invention patent was used to acquire the
changes of physiological activity of wild Acanthopagrus schlegelii in the open sea. Specifically, with this
device, we conducted respiratory metabolism experiments of wild Acanthopagrus schlegelii in the South
China Sea with various sizes (body length 96-122 mm, body weight 9.99-17.36 g) at different temperatures
(16-32℃) and further analyzed the changes of oxygen consumption rate and ammonia excretion rate of
Acanthopagrus schlegelii and its metabolic substrates. It could be seen from the results that the changes of
water temperature have significant effects on the oxygen consumption rate and ammonia excretion rate of
Acanthopagrus schlegelii in the South China Sea. The oxygen consumption rate increases with the rise of
water temperature, while the ammonia excretion rate shows a fluctuating increase. Under the same water
temperature, the oxygen consumption rate and ammonia excretion rate of Acanthopagrus schlegelii were
significantly negatively correlated with weights (P<0.01). Namely, the oxygen consumption rate and
ammonia excretion rate of large Acanthopagrus schlegelii are lower than those of small Acanthopagrus
schlegelii, and the variation of oxygen consumption rate and ammonia excretion rate of Acanthopagrus
schlegelii with different weights conform to the power exponential growth model. The average oxygen
consumption rate and ammonia excretion rate per unit weight of Acanthopagrus schlegelii are 0.11 mg/(g·h)
and 4.48 μg/(g·h) respectively. The change of Q10 coefficient of Acanthopagrus schlegelii shows that the
Q10 value of oxygen consumption rate per unit weight of Acanthopagrus schlegelii is the minimum of 1.31
within the temperature of 24-28℃, and the maximum of 1.69 within 16-20℃. The Q10 value of ammonia
excretion rate is the minimum of 1.05 within 28-32℃, and the maximum of 1.78 within 24-28℃. The O: N
ratio of Acanthopagrus schlegelii in different combinations varies from 15.37 to 44.37. This paper studied the
relationship between important environmental factors affecting the respiratory metabolism of Acanthopagrus
schlegelii and the metabolism of organisms, which is conducive to a better understanding of the metabolic
pattern of Acanthopagrus schlegelii, thus providing basic data for the proliferation and conservation of
Acanthopagrus schlegelii resources.
1 INTRODUCTION
Acanthopagrus schlegelii, which belongs to sparidae
of perciformes, is distributed chiefly in the western
part of the North Pacific Ocean, and also distributed
a
https://orcid.org/0000-0001-6835-2334
b
https://orcid.org/0000-0003-1383-3055
c
https://orcid.org/0000-0003-4657-8185
d
https://orcid.org/0000-0003-2950-0896
in Bohai Sea, Yellow Sea, East China Sea and South
China Sea. Acanthopagrus schlegelii is a eurythermic
and euryhalinous fish species with excellent
adaptability to the environment. Its survival
temperature is within 4.3℃-34.0℃, and the suitable
Feng, X., Fan, J., Zhao, X. and Chen, P.
Study on Respiratory Metabolism of Wild Acanthopagrus schlegelii in South China Sea under Different Temperatures and Weights.
DOI: 10.5220/0011175600003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 5-12
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
5

temperature for its growth is within 17.0℃-25.0℃.
Because of its delicious meat, Acanthopagrus
schlegelii has become an important marine
commercial fish and an excellent species for
aquaculture which has been widely cultivated in
recent years (Lin 2001). Acanthopagrus schlegelii is
an important breeding species with high economic
value and development prospects. In recent years,
studies on its breeding have been increasing and made
some progresses (Liu 2002, Bai 1999, Xu 2008). As
the focus of bioenergy research, respiratory
metabolism is also vital to the study of commercial
species breeding of marine and freshwater fishery
resources and offshore marine pasture. However,
there is few research on respiratory metabolism of
Acanthopagrus schlegelii, especially the wild
individuals. In this paper, the respiratory metabolism
of wild Acanthopagrus schlegelii in the South China
Sea was investigated through indoor experiments in
simulated marine environment. The results can
further enrich the basic data of the study and provide
theoretical guidance and basis for the conservation
and utilization of wild Acanthopagrus schlegelii in
the South China Sea and various coastal areas.
2 MATERIALS AND METHODS
2.1 Experimental Materials
Wild Acanthopagrus schlegelii used for experiments
was caught from the natural waters of Daya Bay in
Shenzhen. Healthy and fresh individuals with body
length of 96 to 122 mm and weight of 9.99 to 17.36 g
were selected. Temporary cultivation was carried out
in a disinfected square pond and then the selected
individuals were moved to the indoor tank for 48 hours
before the experiment. The temperature of seawater in
the temporary pond was 24±0.5℃ with salinity of
30.7±.55, and the natural light cycle was maintained
during the temporary cultivation period by continuous
aeration using oxygenation pumps. The experimental
seawater was taken from the natural seawater of
Dapengao sea area in Daya Bay and was used after
precipitation with salinity of 31.25 and pH of 8.07.
2.2 Experimental Method
The experiment was conducted indoor under natural
illumination, and the experiment started at the same
time every day to ensure consistent experimental
conditions. The experimental device of closed
flowing respiratory metabolism was used for
measuring the relatively static respiratory metabolism
of an organism when it performed respiratory
metabolism and its respiratory chamber was
completely isolated from the outside air. The
experimental device is a self-designed closed flowing
experimental device, which is used to measure the
oxygen consumption rate and ammonia excretion rate
of Acanthopagrus schlegelii (Feng 2018). The water
temperature was controlled by the cold and warm
water exchanger to ensure that the fluctuation of
water temperature was ±0.5℃ during the experiment.
The seawater entered the experimental respiratory
chamber through the panel-type flowmeter, and the
sample water was collected and preserved by the
outlet sampling bottle. During the experiment, the
water velocity was kept at (12±0.4) L/h according to
the physiological characteristics of Acanthopagrus
schlegelii and the pre-experimental results. The
experimental subjects were divided into 10 groups
based on their weights, with 3 parallel samples in
each group. At the same time, 5 water temperature
gradients were set, namely 16℃, 20℃, 24℃, 28℃
and 32℃ respectively. When the Acanthopagrus
schlegelii adapted to the environment and the water
flow became stable after a period of time, the water
samples were collected from the outlet and fixed on
the spot to measure the dissolved oxygen value, and
the ammonia nitrogen value was taken under the
temperature of -20℃ and then the Acanthopagrus
schlegelii was kept in the laboratory for
measurement. After sampling, the Acanthopagrus
schlegelii was taken out from the experimental
device, drained and dried in an electric thermostatic
oven at 60℃ to the constant weight to measure the
body length and weight. Dissolved oxygen (DO) was
measured by iodometric method, and ammonia
nitrogen was measured by FIAstar™ 5000 flow
injection analysis system of FOSS. The sodium
hypobromite oxidation method in the marine survey
specification (GB 1274-2007) was also used for
testing and calibration. Each experimental sample
was measured twice, and the average value of three
groups of parallel samples was taken.
2.3 Analysis Method
2.3.1 Calculation Formula for Oxygen
Consumption Rate and Ammonia
Excretion Rate
𝑄
=
×
(
)
(1)
𝑄
=
×(
)
(2)
Formula (1) is for the calculation of oxygen
consumption rate, and Formula (2) is for the
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
6

calculation of ammonia excretion rate. In Formulae
(1) and (2), Q
0
is oxygen consumption rate (mg/g•h),
Q
T
is ammonia excretion rate (μg/g•h), W is the body
weight (g), V is the water velocity (L/h), A
1
is the
initial dissolved oxygen (mg/L), A
2
is the dissolved
oxygen (mg/L) after a period of time, N
0
is the initial
ammonia and nitrogen concentration (ug/L), and N
T
is
the ammonia and nitrogen concentration (μg/L) after a
period of time.
2.3.2 Calculation Formula for Metabolic
Impact Intensity
𝑄
=(
)
(
)
(3)
In Formula (3), Q
10
is the intensity of the effect of
water temperature on metabolism, indicating the
change rate of respiratory metabolism of
acanthopagrus schlegelii for every 10℃ increase in
water temperature. M
1
and M
2
are the metabolic rates
of acanthopagrus schlegelii at temperatures T
1
and T
2
respectively.
2.3.3 Calculation Formula for the Analysis
of Respiratory Metabolic Substrates
𝑂: 𝑁 = 1000 ×
(4)
In Formula (4), Q
0
and Q
T
are oxygen consumption
rate and ammonia excretion rate at the same
temperature, respectively.
3 RESULTS AND ANALYSIS
3.1 Relationship between Respiratory
Metabolism and Water
Temperature of Acanthopagrus
Schlegelii
The results of oxygen consumption rate of
Acanthopagrus schlegelii at different temperatures are
shown in Fig. 1. Within the temperature ranging from
16 to 32℃, the oxygen consumption rate of
Acanthopagrus schlegelii increases with the rise of
water temperature. According to the figure of the
relationship between oxygen consumption rate of
Acanthopagrus schlegelii and the water temperature,
the oxygen consumption rate of Acanthopagrus
schlegelii with certain body weight has no obvious
change, when the experimental water temperature
ranges from 24 to 32℃. This might be related to the
temperature suitable for the survival of
Acanthopagrus schlegelii. The oxygen consumption
rate of black seabream is the maximum at the
experimental water temperature of 32°C. The one-way
ANOVA test shows that the effect of temperature on
the oxygen consumption rate of Acanthopagrus
schlegelii reached a highly significant level
(F=18.231, P<0.01) within the range of experimental
water temperature.
Within the experimental water temperature
ranging from 16 to 32℃, the variation of ammonia
excretion rate and oxygen consumption rate of
Acanthopagrus schlegelii are similar (as shown in
Fig. 2). However, with the rise of water temperature,
the ammonia excretion rate of Acanthopagrus
schlegelii demonstrates a fluctuating increase in the
experimental water temperature ranging from 24 to
32℃. It is possible that the high temperature affects
the excretion of Acanthopagrus schlegelii thus
causing disorder. The one-way ANOVA test revealed
that the effect of temperature on ammonia excretion
rate of Acanthopagrus schlegelii reached a significant
level (F=3.496, P<0.05) within the range of
experimental water temperature.
The results of average oxygen consumption rate
and ammonia excretion rate per unit of weight of
Acanthopagrus schlegelii at different temperatures
also indicates that the oxygen consumption rate and
ammonia excretion rate shows a fluctuating increase
as the water temperature keeps rising (as shown in
Tab. 1). The average oxygen consumption rate per
unit of weight of Acanthopagrus schlegelii is 0.11
mg/g·h and its ammonia excretion rate per unit of
weight is 4.48 μg/g·h when the experimental water
temperature is 16℃, 20℃, 24℃, 28℃ and 32℃,
respectively.
Figure 1: Variation Relationship between oxygen
consumption rate and water temperature of Acanthopagrus
schlegelii.
Figure 2: Variation Relationship between ammonia
excretion rate and water temperature of Acanthopagrus
schlegelii.
Study on Respiratory Metabolism of Wild Acanthopagrus schlegelii in South China Sea under Different Temperatures and Weights
7
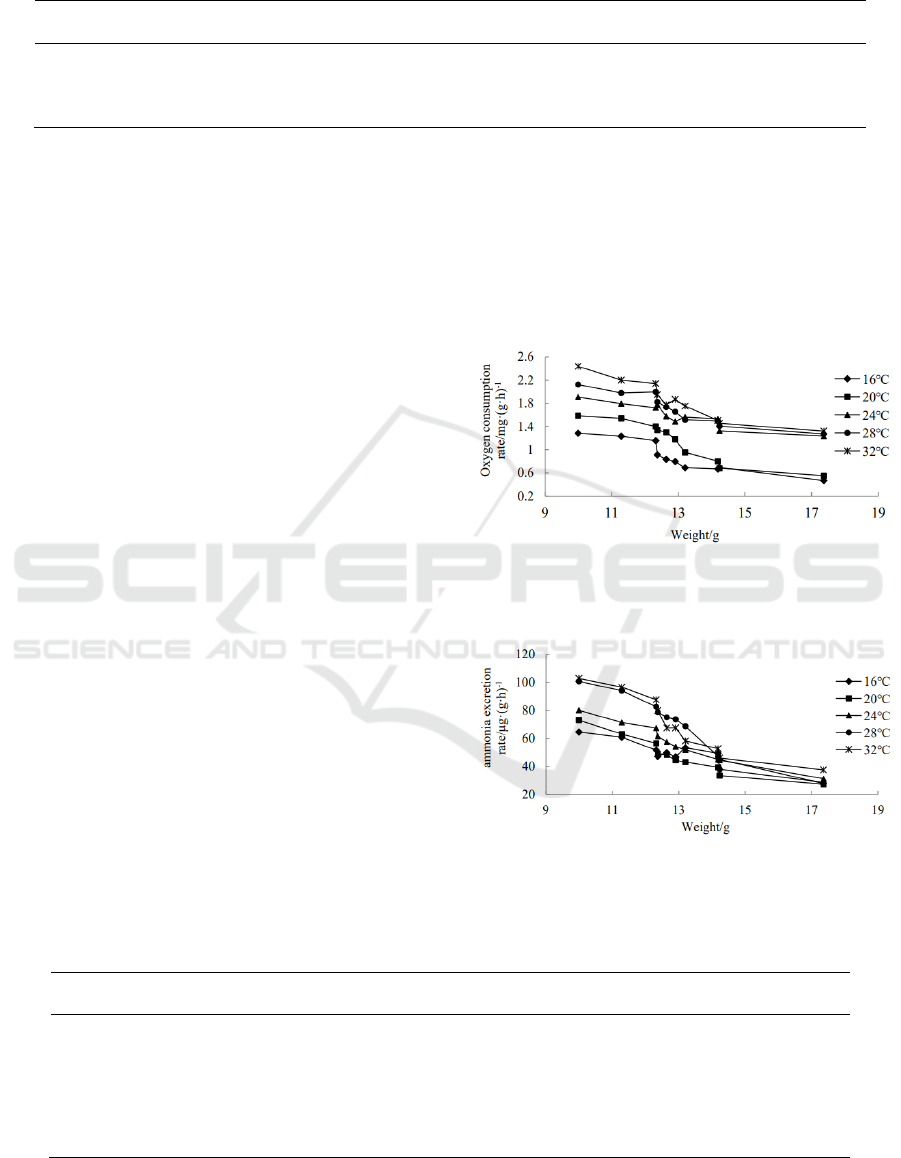
Table 1: Oxygen consumption rate and ammonia excretion rate per unit of weight under different water temperature of
Acanthopagrus schlegelii.
Experimental water temperature
(
℃
)
16 20 24 28 32
Avera
g
e value
Oxygen consumption rate per unit
of weight (mg/g•h)
0.07 0.09 0.12 0.13 0.14 0.11
Ammonia excretion rate per unit of
weight (μg/g•h)
3.81 3.66 4.33 5.27 5.34 4.48
3.2 Relationship between Respiratory
Metabolism and Weight of
Acanthopagrus Schlegelii
Under the same water temperature, the results of
oxygen consumption rate of Acanthopagrus
schlegelii with different weights are shown in Fig. 3.
With the increase of experimental individuals, the
oxygen consumption rate of Acanthopagrus
schlegelii keeps decreasing, i.e., they show a negative
correlation. The correlation analysis shows that there
is a significant negative correlation between the
weight and oxygen consumption rate of
Acanthopagrus schlegelii at the level of 0.01
(P<0.01) within the range of experimental water
temperature. Under different water temperatures, the
change of oxygen consumption rate of
Acanthopagrus schlegelii with different weights is
consistent to the power exponential growth model (as
shown in Tab. 2). The range of value a is 3.6459 to
10.129 and the range of value b is -0.172 to -0.079 in
the change of oxygen consumption rate of
Acanthopagrus schlegelii at the water temperature of
16 to 32℃.
The results of ammonia excretion rate are similar
to oxygen consumption rate. The ammonia excretion
rate of small Acanthopagrus schlegelii is higher than
that of large Acanthopagrus schlegelii, and the larger
the Acanthopagrus schlegelii, the smaller the
ammonia excretion rate per unit weight (Fig. 4). The
correlation analysis shows that there is a significant
negative correlation between body weight and
ammonia excretion rate of Acanthopagrus schlegelii
at 0.01 level (P < 0.01). Under different water
temperatures, the change of ammonia excretion rate
of Acanthopagrus schlegelii with different weights is
consistent with the power exponential growth model
as shown in Tab. 3. The range of value a is 197.4 to
843.32 and the range of value b is -0.197 to -0.107 in
the change of ammonia excretion rate of
Acanthopagrus schlegelii.
Figure 3: Variation Relationship between oxygen
consumption rate and body weight of Acanthopagrus
schlegelii.
Figure 4: Variation Relationship between ammonia
excretion rate and body weight of Acanthopagrus
schlegelii.
Table 2: The relationship between oxygen consumption rate and body weight of Acanthopagrus schlegelii.
Temperature
(℃)
Equation R
2
a b
16 y = 6.0098e
-0.151x
0.8807 6.0098 -0.151
20 y = 10.129e
-0.172x
0.8677 10.129 -0.172
24 y = 3.6459e
-0.064x
0.8476 3.6459 -0.064
28 y = 4.7016e
-0.079x
0.8621 4.7016 -0.079
32 y = 6.1059e
-0.093x
0.8834 6.1059 -0.093
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
8

Table 3: The relationship between ammonia excretion rate and body weight of Acanthopagrus schlegelii.
Temperature
(℃)
Equation R
2
a b
16 y = 197.40e
-0.107x
0.8720 197.40 -0.107
20 y = 296.11e
-0.143x
0.9321 296.11 -0.143
24 y = 324.32e
-0.136x
0.9681 324.32 -0.136
28 y = 843.32e
-0.197x
0.8935 843.32 -0.197
32 y = 518.85e
-0.157x
0.8929 518.85 -0.157
3.3 Changes in Q
10
Coefficients Caused
by the Metabolism of
Acanthopagrus Schlegelii
The effect of water temperature on the respiratory
metabolism of Acanthopagrus schlegelii could be
expressed by Q
10
value (Tab. 4). The results of
different experimental water temperatures indicates
that the Q
10
value of oxygen consumption rate per unit
weight of Acanthopagrus schlegelii is the minimum
of 1.31 within the temperature of 24 to 28℃; and the
maximum of 1.69 within the temperature of 16 to
20℃. The Q
10
value of ammonia excretion rate per
unit weight of Acanthopagrus schlegelii is the
minimum of 1.05 within the temperature of 28 to
32℃, and the maximum of 1.31 within the
temperature of 24 to 28℃.
3.4 Analysis of Respiratory Excretion
Substrate
The relationship between weight, water temperature
and O: N of Acanthopagrus schlegelii was
investigated by the analysis of respiratory excretion
substrate. The O: N ratio of Acanthopagrus schlegelii
under different water temperatures varies (Fig. 5), and
the variation of O: N ratio of Acanthopagrus
schlegelii ranges from 15.37 to 44.37, and the O: N
ratio of metabolic substrate is the minimum at 16℃
and the maximum at 28℃. In small Acanthopagrus
schlegelii and the large Acanthopagrus schlegelii
with the experimental water temperature below 24℃,
the energy-supplying substances of respiratory
excretion are a mixture of protein and fat. When the
experimental water temperature reached 24℃ or even
higher, the energy-supplying substances in the
excretion substrate of large Acanthopagrus schlegelii
are mainly a mixture of fat and carbohydrate.
Table 4: The Q
10
coefficients of Acanthopagrus schlegelii
ammonia excretion rate.
Temperat
ure (℃)
Oxygen consumption
rate Q
10
Ammonia
excretion
rate Q
10
16~20 1.69 1.36
16~24 1.64 1.31
16~28 1.52 1.45
16~32 1.49 1.34
20~24 1.59 1.26
20~28 1.44 1.49
20~32 1.43 1.33
24~28 1.31 1.78
24~32 1.36 1.37
28~32 1.41 1.05
Figure 5: The relationship between body weight, water
temperature and O: N on Acanthopagrus schlegelii.
4 DISCUSSIONS
Water temperature is an important environmental
factor affecting the respiratory metabolism of fish,
and its fluctuation is the key focus of biological
respiratory metabolism (Spanopoulos-Hernández
2005). The oxygen consumption rate varies among
different species. Generally speaking, the oxygen
consumption rate and the water temperature are
positively correlated within a certain range of water
temperature (Song 1997, Wang 2002). The results of
oxygen consumption rate of Acanthopagrus
schlegelii reveal that its average oxygen consumption
rate is lower than that of certain fish in the same area,
Study on Respiratory Metabolism of Wild Acanthopagrus schlegelii in South China Sea under Different Temperatures and Weights
9

which is related to the maturity of fish and
interspecific genetic factors. The oxygen
consumption rate increases with the rise of water
temperature. As the water temperature rises, the
activity performance and biochemical reaction speed
of animal tissues and organs also increase, which
leads to the acceleration of respiration and excretion.
This is the common feature of ectotherm (Wang
2010). The external temperature at which the
maximum respiratory metabolism occurs is the
optimum living temperature of the organism. The
fitted curve of the effect of water temperature change
on the respiratory metabolism of Acanthopagrus
schlegelii studied in this paper basically leveled off at
28℃. At this temperature, the physiological activity
of Acanthopagrus schlegelii reached its peak and the
respiratory metabolic rate was fast, indicating that the
temperature at around 28℃ might be the optimum
temperature for Acanthopagrus schlegelii. In the
experiment of Acanthopagrus schlegelii, the results
are similar to those of Zheng Jianmin et al. on juvenile
acanthopagrus schlegelii at 17.5 to 21.0℃, and the
experimental subjects are farmed Acanthopagrus
schlegelii (Zheng 1991). We can speculate that there
is little difference in oxygen consumption rate
between farmed and wild Acanthopagrus schlegelii.
The change of ammonia excretion rate of
Acanthopagrus schlegelii with water temperature is
similar to that of oxygen consumption rate, which
indicates that the change of metabolism in
Acanthopagrus schlegelii caused by temperature is
also influenced by enzyme activity and activity of
internal body organ. As the water temperature rises,
the basal metabolism of Acanthopagrus schlegelii
also increases, showing the enhancement of body
excretion. In the excretion study of hybrid
Acanthopagrus schlegelii and juvenile
Acanthopagrus schlegelii, Yan Fuyun et al. found that
the ammonia excretion rate increases with the rise of
water temperature in juvenile hybrid Acanthopagrus
schlegelii and juvenile Acanthopagrus schlegelii at
water temperature from 13 to 28°C (Yan 2010).
Organisms control energy metabolism through the
regulation of biological functions by weight. The
results show that the oxygen consumption rate of
Acanthopagrus schlegelii decreases with the increase
of weight, which may be related to the change of the
proportion of tissues that sustain the life of fish in the
body. Tissues are used to sustain the life of fish, such
as: brain, kidney, and gonads, have high oxygen
consumption. While tissues do not directly sustain
life, such as bones, muscles, and fats, have low
oxygen consumption (Li 2009). Small fish under
growth and development stage usually has large
proportion of tissue in the front part and small
proportion of tissue in the back part. In contrast, large
fish often has small proportion of the front part and
large proportion of the back part. For this reason, the
metabolic activity of small fish is more vigorous than
that of large fish (Wang 2011). The oxygen
consumption rate of Acanthopagrus schlegelii
decreases successively with the increase of weight,
which is similar to that of the fry of Fugu obscurus
(Wang 2002) and Perca fkuviatilis (Zakęś 2003).
There is a negative correlation between ammonia
excretion rate and the weight of fish. Since mature
fish are well-developed with strong anti-interference,
external changes have less impact on them than that
of juvenile fish. This was also found in other aquatic
organisms such as Strongylocentrotus intermedius
(Bi 2000), Penaeus japonicas (Zhu 2001),
Oratosquilla oratoria (Jiang 2000) and Apostichopus
japonicas (Sun 2012), suggesting a more pronounced
effect of weight on respiratory metabolism.
The value of Q
10
reflects the extent to which the
metabolic intensity is affected by temperature (Bayne
1983), which indicates the change in oxygen
consumption rate caused by every 10°C increase in
water temperature. The Q
10
value of oxygen
consumption rate of Acanthopagrus schlegelii varies
from 1.31 to 1.69. The larger the Q
10
value, the more
sensitive it is to the change of water temperature
within such temperature range, and the oxygen
consumption rate changes significantly. The Q
10
value of ammonia excretion rate varies from 1.05 to
1.78, and high Q
10
values indicates that there is an
upper limit exceeding the temperature tolerance
threshold of experimental fish in this temperature
range, which affects its physiological activities.
The energy substances metabolized by animals
are proteins, fats and carbohydrates, which are
eventually metabolized into CO
2,
water and nitrogen
while releasing energy. Excretion is one of the basic
physiological activities of energy metabolism in
organisms. The ratio of metabolic substrate O: N can
be used to deduce the source of energy substances.
Changes in O: N ratio are closely related to the
environmental factors to which the organisms are
subjected and can be used to determine the growth of
organisms under specific conditions (Widdows
1978). When the energy supply of the body is
provided by proteins, the O: N ratio is about 7 to 10.
When proteins and fats are oxidized for energy
supply, the O: N ratio is about 24. An infinitely
increasing O: N ratio is presumed to be a combined
energy supply of fats and carbohydrate (Mayzaud
1978, Ikeda 1974, Conover 1968). In the
experimental results, the O: N ratio of metabolic
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
10

substrates of Acanthopagrus schlegelii suggests that
the metabolic energy substances are supplied by a
mixture of protein and fat, together with a small
amount of carbohydrates. This is consistent with the
research on basic metabolic law of juvenile
Paralichthys olivaceus by Wang Bo et al. Their
research results show that the average O: N ratio of
metabolic substrate of juvenile Paralichthys
olivaceus at different temperatures is 38.8, and the
main energy supply is a nutrient mixture such as
proteins and fats, followed by some carbohydrates
(Wang 2004). When the O: N ratio of metabolic
substrate reaches the maximum, the mixed metabolic
difference of proteins, fats and sugars in aquatic
organisms is the largest, and the growth rate is the
highest. Many scholars regard the O: N ratio of
substrate as an indicator of biological adaptation to
the environment and the determination of suitable
conditions (Xu 2008). In this study, the O: N ratio of
Acanthopagrus schlegelii fluctuates significantly
with water temperature, and the results reveal that the
growth rate of Acanthopagrus schlegelii is the highest
at 28℃, indicating that this temperature is the
optimum for the growth of Acanthopagrus schlegelii
under experimental conditions.
5 CONCLUSIONS
The growing market demand for aquatic products has
stimulated the development of aquaculture in the
whole world, China included. Due to overfishing and
marine environmental pollution, China’s aquaculture
production continues to decline, bringing great
environmental pressure to the aquaculture industry.
Since Acanthopagrus schlegelii has high economic
value, it is of great importance to reasonably protect,
utilize and vigorously farm it. The study on the
respiratory metabolism of wild Acanthopagrus
schlegelii in this article fills the gap in the research on
the respiratory metabolism of wild Acanthopagrus
schlegelii in the South China Sea and enriches the
data on its growth. It provides reference for the
assessment of the resources of Acanthopagrus
schlegelii and snapper, the growth of offshore
Acanthopagrus schlegelii aquaculture and the
assessment on the proliferation capacity of offshore
marine pastures.
ACKNOWLEDGEMENTS
This work was financially supported by the Applied
Research Project of Guangdong Province R&D
Project in Key Areas (2020B1111030002); National
Key Technology R&D Program (2012BAD18B02);
Basic Research Business Expenses Project of Chinese
Academy of Fishery Sciences (2020TD06).
REFERENCES
Bai Huaiping. Food Habit of Black Pargy (Sparus
marocephalus) in Xiangshan Port [J]. Journal of Ningbo
University (Natural Science and Engineering Edition),
1999,12 (4):42-47.
Bayne B, Newell R. (1983). Physiological energetics of
marine molluscs. J. The mollusca. 4(1):407-415.
Bi Y, Jiang S, Liu H, Xue K, Wang N, Wang C. (2000).
Effect of Temperature and Weight on Oxygen
Consumption Rate and Ammonia Excretion Rate of
Strongylocentrotus Intermedius. J. Fisheries Science,
(4):5-7.
Conover R J, Corner E. (1968). Respiration and nitrogen
excretion by some marine zooplankton in relation to
their life cycles. J. Journal of the Marine Biological
Association of the United Kingdom, 48 (1):49-75.
Feng Xue, Zhou Yanbo, Fan Jiangtao, Yu Jing, Yuan
Huarong, Wang Wenjie, Chen Pimao. (2018).
Combined effect of body mass and water temperature
on oxygen consumption, nitrogen excretion rate of
starved Mugil cephalus. J. South China Fisheries
Science, 14 (1):114-120.
Ikeda T. (1974). Nutritional ecology of marine
zooplankton. J. Memoirs of the Faculty of Fisheries
Hokkaido University, 22 (1):1-97.
Jiang Zuhui, Wang Jun, Tang Qisheng. (2000). Studies on
Effects of Body Weight, Water Temperature and
Starvation on Respiration and Excretion of Mantis
Shrimp (Oratosquilla Oratoria). J. Progress in Fishery
Sciences, (3):28-32.
Li Jiaer, Liu Shirui, Ou Youjun, Zhang Jiansheng, Tao
Qiyou, Guo Genxi. (2009). Respiratory and Excretory
Metabolism of Fish Fry of Yellow-spotted Grunt
Plectorhynchus cinctus. J. South China Fisheries
Science, 5 (2):34-39.
Lin Jinbiao, Chen Tao, Chen Lin, Guo Jinfu. (2001). The
techniques of Sparus marocephalus tagged and released
in Daya Bay. J. Journal of Fisheries of China, (1):79-
83.
Liu Duanwei, Zhao Guangmiao, Ren Quanna. (2002).
Breeding Technologies of Sparidae- Breeding
Technology of Acanthopagrus Schlegelii in Seawater
Pond. J. China Fisheries, (9):53,62.
Mayzaud P. (1976). Respiration and nitrogen excretion of
zooplankton. IV. The influence of starvation on the
metabolism and the biochemical composition of some
species. J. Marine Biology, 37 (1):47-58.
Study on Respiratory Metabolism of Wild Acanthopagrus schlegelii in South China Sea under Different Temperatures and Weights
11

Song Suxiang, Liu Hongbai. (1997). The asphyxiation
Point and oxygen consumption rate of Acipenser
Schrenckii. J. Journal of Fishery Sciences of China,
4(5):100-103.
Spanopoulos-Hernández M, Martínez-Palacios C A,
Vanegas-Pérez R C, Rosas C, Ross L G. (2005). The
combined effects of salinity and temperature on the
oxygen consumption of juvenile shrimps Litopenaeus
stylirostris (Stimpson,1874). J. Aquaculture, 244(1):
341-348.
Sun Zhenlong. (2012). Study on the Main Nutrient
Metabolism of the Farming Sea Cucumber. D. Ocean
University of China.
Wang Bo, Li Jiqiang, Cao Zhihai, Li Dejun, Sun Qingxia,
Zhu Mingyuan, Mao Xinghua. (2004). A Preliminary
Study on Standard Metabolism of Juvenile Summer
Flounder (Paralichthys dentatus). J. Advances in
Marine Science, (1):62-68.
Wang Jun, Jiang Zuhui. (2002). Study on Oxygen
Consumption Rate and Ammonia Excretion Rate of
Chlamys Farreri. J. Chinese Journal of Applied
Ecology, 13 (9):1157-1160.
Wang Peijun, Zhao Qingliang, Yin Ning, Gu Shuyu.
(2002). Preliminary Study on Oxygen Consumption
Rate and Asphyxiation Point of Fugu Obscurus Fry. J.
Journal of Hydroecology, (6):3-4.
Wang Xingqiang, Cao Mei. (2010). Effects of Low Salinity
and Low Temperature on Growth and Energy Budget
of Juvenile Exopalaemon carinicauda. J. Journal of
Hydroecology, 3 (2):66-71.
Wang Zisheng, Guo Xijie, Huang Jintian, Qi Zhitao, Peng
Bin, Wang Aimin. (2011). Effect of Salinity and Body
Mass on Standard Metabolic Rates of Cynoglossus
Semilaevis. J. Marine Sciences, 35 (3):83-86.
Widdows J. (1978). Physiological indices of stress in
Mytilus edulis. J. Journal of the Marine Biological
Association of the United Kingdom, 58 (1):125-142.
Xu Hailong, Liu Haiying, Lin Yuejiao. (2008). Effect of
Temperature and Salinity on Respiration of Mantis
Shrimp (Oratosquilla Oratoria). J. Fisheries Science,
27 (9):443-446.
Xu Kaida, Zhou Yongdong, Wang Weiding, Xue Lijian,
Zhang Hongliang, He Zhouting, Pan Guoliang. (2008).
The tagging and releasing experiment of Sparus
microcephalus ( Basilewsky) in the Zhoushan sea area.
J. Journal of Shanghai Fisheries University, (1):93-97.
Yan Fuyun, Xu Shanliang, Gu Jiangwen, Chen Xuanxiong,
Lyu Huiming, Jia Chaoyan. (2010). Comparison of
Metabolic and Excretion Rates of Young Sparus
Macrocephalus and Young Hybrid Porgy. J. Journal of
Oceanography in Taiwan Strait, 29 (4):496-502.
Zakęś Z, Demska-zakęś K, Kata K. (2003). Rates of oxygen
consumption and ammonia excretion of juvenile
Eurasian perch Perca fluviatilis. J. Aquaculture
International, 11(3):277-288.
Zheng Jianmin, Li Jiaer, Ou Youjun. (1991). Preliminary
Study on Oxygen Consumption of Juvenile of Black
Porgy Sparus Macrocephalus (Basilewsky). J. Marine
Science Bulletin, 10 (4):47-51.
Zhu Xiaoming, Wu Lisheng, Ma Zhiyong, Li Shaojing.
(2001). Primary Studies on Respiration and Excretion
of Penaeus Japonicus post-larvae. J. Journal of
Oceanography in Taiwan Strait, (1):37-42.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
12
