
Effect of Microplastics on Gut Bacterial Community of the
Earthworm Pheretima guillelmi
Qiongqiong Shang
a
, Mingxia Tan
and Jie Chi
School of Environmental Science and Engineering, Tianjin University, Tianjin, 300350, PR, China
Keywords: Earthworm, Microplastics, Intestinal.
Abstract: Earthworm is an important part of the soil ecosystem. Study on the responses of earthworm gut bacteria to
microplastics (MPs) is still lacking. In this work, the effects on non-biodegradable polyethylene (PE) and
biodegradable poly (butylene adipate-co-terephthalate) (PBAT) MPs on bacterial community of earthworm
gut were investigated. The results showed that the number of operational Taxonomic Units (OTUs) and α
diversity indexes in treatments with MPs were higher than those in treatment without MPs. The number of
OTUs was in the order of treatments with aged-PE MPs > treatments with unaged-PE MPs > treatments with
PBAT MPs. The number of OTUs in treatments with lower MPs concentration was higher than that in
treatments with higher MPs concentration. Addition of MPs increased the relative abundances of genera
Ensifer, Bacteroides and Bacillus, but decreased the relative abundances of genera Salmonella, Escherichia-
Shigella and Paracoccus. Therefore, MPs significantly impact the microbial community of earthworm gut,
which is related to types of MPs.
1 INTRODUCTION
1
Soil animals are widely distributed all over the world
and play a key role in soil health and biodiversity
(BARDGETT 2014). Among them, earthworms are
the largest invertebrates in the soil, which can
enhance soil structure and fertility (BERTRAND
2015). Most of the ecological functions of
earthworms are connected to their internal microbial
communities, which are very sensitive to external
environmental interference (ZHANG 2022).
However, our research on the intestinal microflora of
soil animals has just begun, and the understanding of
the composition diversity and ecological functions of
the earthworms gut bacteria is still lacking, and
further research is required.
Microplastics (MPs) are widely present in the soil
environment, and absorb organic pollutants and metal
pollutants in the soil, even have a certain impact on
animals and microorganisms in soils. At the same
time, exposure to MPs disturbs the growth of
earthworms (HUERTA 2016), thereby affecting their
intestinal microbial community. However, the
concentration and properties of MPs are different, and
the effects on earthworms are also distinct.
a
https://orcid.org/0000-0002-1939-4009
Therefore, in this research, two types of MPs,
non-biodegradable polyethylene (PE) and
biodegradable poly (butylene adipate-co-
terephthalate) (PBAT), were selected and earthworms
(Pheretima guillelmi) were the target species to study
the effects of different types and different amounts of
MPs particles on the structure of the earthworm gut
bacterial community.
2 MATERIALS AND METHODS
2.1 Preparation and Characterization
of Materials
Polyethylene (PE) and poly (butylene adipate-co-
terephthalate) (PBAT)with particle size ranges of
104-178 μm are purchased from Dongguan Plastic
Technology Co. Ltd. The MPs were washed with
methanol for 48 hours (changing methanol every 24
hours), and then dried in a fume hood at room
temperature. Aged PE (APE) was prepared by 18%
(v/w) H
2
O
2
and UV exposure (HUFFER 2018). The
functional groups of MPs were determined by FTIR
260
Shang, Q., Tan, M. and Chi, J.
Effect of Microplastics on Gut Bacterial Community of the Earthworm Pheretima guillelmi.
DOI: 10.5220/0011199300003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 260-264
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
spectrometer (Varian Excalibur HE 3100). The
carbonyl index (CI) is often used as an indicator of
the presence of carbonyl groups (SONG 2017). The
calculation of CI of three types of MPs was
determined based on the absorbance at 1720, 2850
cm
-1
: CI=Abs (1720 cm
-1
) / Abs (2850 cm
-1
). The
crystallinity of MPs was determined by X-ray
diffraction (XRD) of Bruker company.
The soil was collected from the Beiyang Park
Campus, Tianjin University, China. After air-dried,
the soil samples were sieved through a 2-mm mesh.
The basic physicochemical properties of the soil
samples were as follows: pH 8.08, total organic
content 1.50%, and composition of 6.09% sand,
68.33% silt and 25.58% clay.
The earthworm Pheretima guillelmi was selected
for this work. The earthworms were first incubated in
soil for 7~14 days to adapt to the laboratory
conditions, maintaining soil moisture content at
70%~85% during culture. Before use, healthy
earthworms were selected and placed on hydrated
filter paper at 20 ±3 °C for 24 h to clear their gut
contents.
2.2 Experiments Methods
There seven treatments were set up, including soils
without MPs (CK), soil +0.2% (w/w) MPs
(i.e.PE,APE and PBAT) and soil +2% (w/w) MPs
(i.e.PE,APE and PBAT). Portions of 200 g soil with
or without MPs were added into a series of 500 mL
glass beakers (distilled water was added into the
beakers to keep soil moisture content of 85%). Each
treatment was replicated 3 times. Five healthy
earthworms (have obvious reproductive ring, sexual
maturity) were washed with distilled water, weighed
and added into the beakers, which covered gauze to
prevent the earthworm escaping. These beakers were
placed in an intelligent illumination incubator (3200
Lux) with 12 h light/12 h dark at 20 ±3 °C for 28 days.
The earthworms were removed from soil on day 28,
washed with distilled water and placed in alcohol.
After being inactivated, the earthworm was dissected
and the gut was removed to measure the microbial
community structure (MA 2017).
3 RESULTS AND DISCUSSIONS
3.1 Characterization of Microplastics
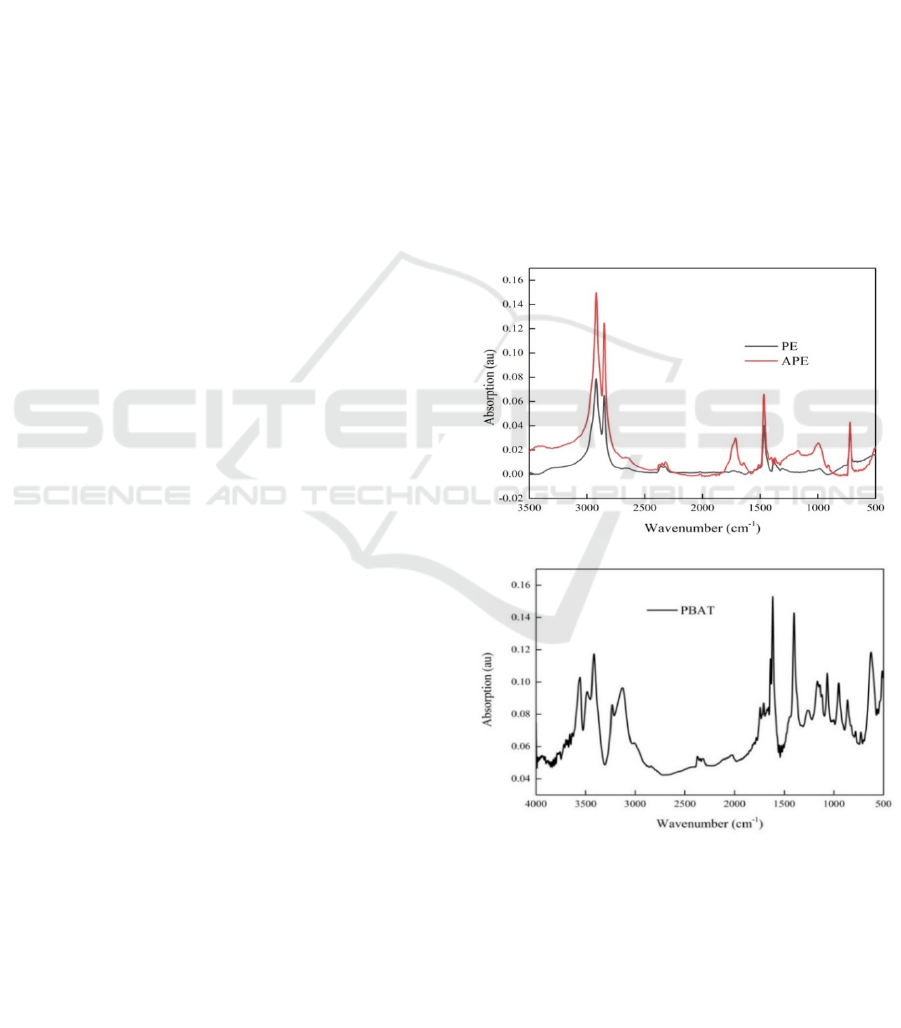
The FTIR fingerprints appear to be quite different for
MPs derived (Fig. 1). Compared to the spectra of PE
and APE, the APE has an obvious peak in the
carbonyl region (1870~1540cm
-1
). The CI increased
from 0.04 (PE) to 0.27 (APE). It is indicating that the
polarity of the PE increased after aging. In addition,
the peak of PBAT spectra in the carbonyl region is
more obvious, and the CI of PBAT is 0.92, and the
polarity is the strongest.
According to the degree of crystallinity, MPs can
be divided into crystalline state, semi-crystalline state
and amorphous state (amorphous). Amorphous MPs
also include glassy state and rubber state (GUO
2012). The X-ray diffractograms of PE/APE/PBAT
composites are shown in Fig.2. The crystallinity of
MPs varies. As semi-crystalline plastics, PE has
obvious crystallization peaks, APE also shows
obvious crystallization peaks. Through jade data
processing, PE crystallinity is 56.85%, while APE is
61.67%. Compared with PE, the crystallinity of APE
increased slightly. PBAT has no obvious
crystallization peak and is a rubber polymer.
Figure1: FTIR spectra of microplastics.
Effect of Microplastics on Gut Bacterial Community of the Earthworm Pheretima guillelmi
261
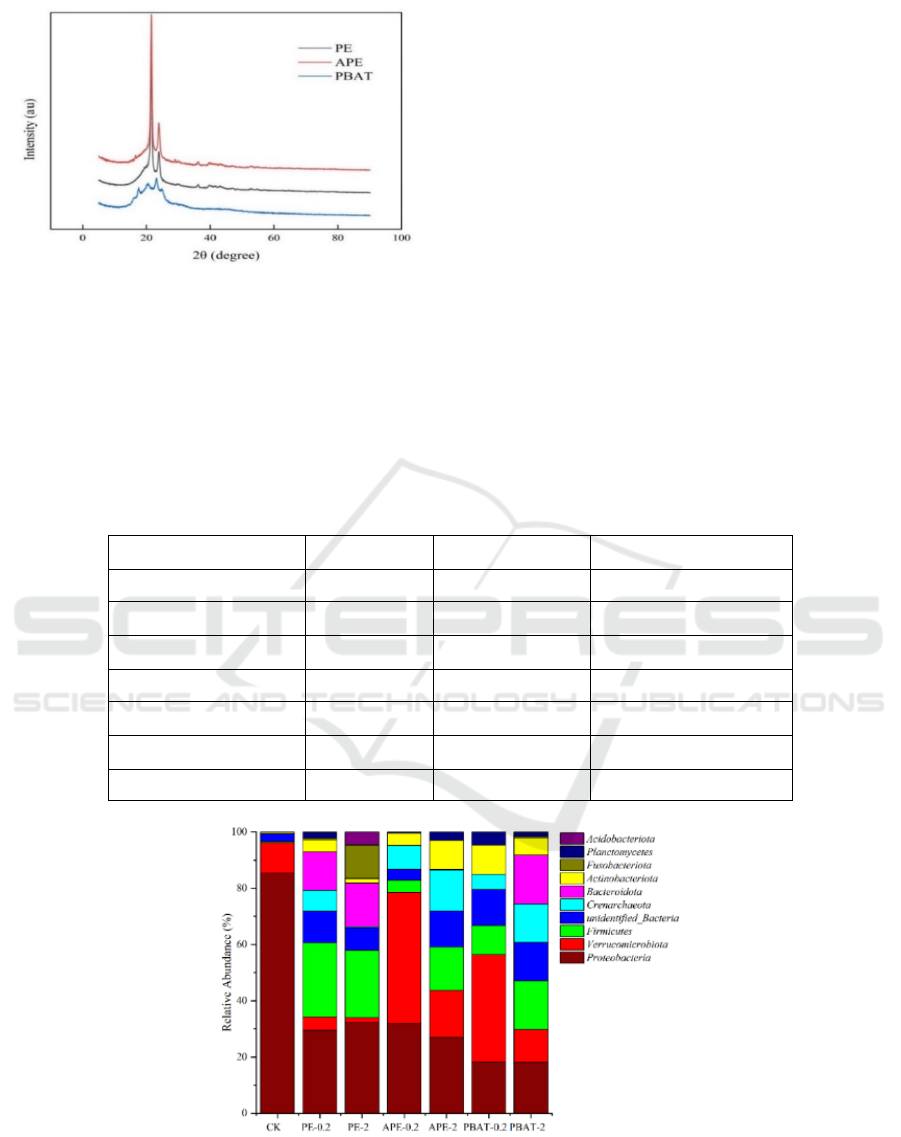
Figure 2: XRD of microplastics.
3.2 Effects of Microplastics on
Earthwormsgut Bacteria
At the end of the experiment, 16SrDNA sequences
ofthe samples of earthworms guts have been tested,
and the results of OTUs analysis were as shown in
Table 1. The results show that, compared with the
CK, the number of OTUs in treatments with MPs
significantly increased. The number of OTUs of the
low concentration treatments were higher than that of
the high at the same type of MPs. The variation trend
of α diversity index (Shannon and Simpson) of the
earthworm gut bacterial community was essentially
consistent with the number of OTUs.
Figure 3 shows the top 10 phyla in the relative
abundances of the earthworm gut bacteria. It can be
seen that the relative abundances of
Verrucomicrobiota and Firmicutes in the earthworm
gut were the highest for all cultivation. Compared
with CK, the relative abundances of Firmicutes,
Crenarchaeota, Bacteroidota, Actinobacteriota and
Planctomycetes increased significantly in those
treatments with MPs with the highest increase of
Firmicutes by 3.28~23.78%, while the relative
abundances of Proteobacteria significantly reduced
by 53.70%~69.10%. And the relative abundances of
Bacteroidota increased when the concentration of
MPs increased.
Table 1: The number of OTUs and α diversity index of earthworm gut bacteria.
Group OTUs Shannon Simpson
CK 357 2.135 0.588
PE-0.2 1774 7.297 0.979
PE-2 1163 6.768 0.982
APE-0.2 945 4.045 0.829
APE-2 778 5.952 0.958
PBAT-0.2 764 5.534 0.939
PBAT-2 752 7.034 0.973
Figure 3: Thebacterial community composition of earthworm gut is shown as relative abundance (%) at the phylum levels.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
262
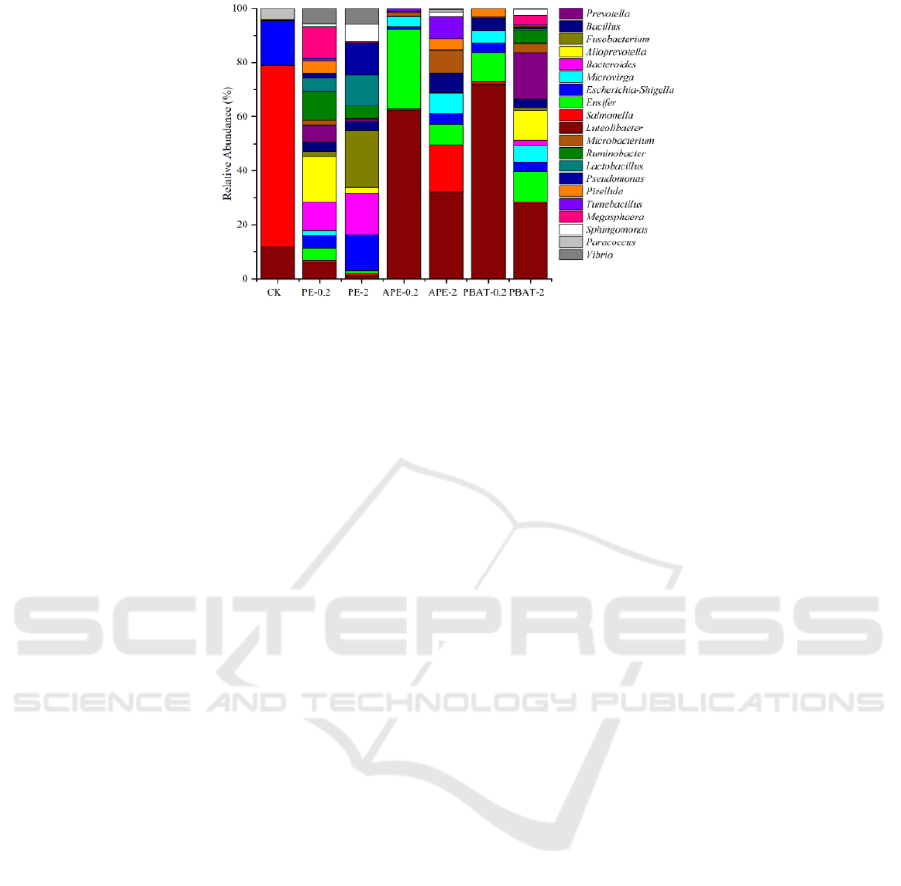
Figure 4: The bacterial community composition of earthworm gut is shown as relative abundance (%) at the genera levels.
Figure 4 lists the top 20 bacterial genera in the
relative abundance of the earthworm gut bacteria,
belonging to 6 phyla, namely Verrucomicrobia,
Proteobacteria, Actinobacteria, Planctomycetes,
Bacteroidetes and Firmicutes. Additionally,
compared with CK, the relative abundance of Ensifer,
Bacteroides, and Bacillus in the earthworm gut
increased significantly after added MPs, while the
relative abundances of Salmonella, Escherichia-
Shigella and Paracoccus decreased significantly.
Many studies have reported that earthworms
produce more intestinal secretions when they ingest
materials without rich fresh organic matter.
Therefore, MPs addedto the soil may stimulate
earthworms to produce more intestinal mucus.
Simultaneously, the bacteria in the gut of earthworms
is stimulated, which has a significant effect on the
consumption of pollutants (HUERTA 2016). In this
study, the number of OTUs and α diversity indexes of
the earthworm gut bacteria increased significantly
after adding MPs to the soil where earthworms live.
Addition of MPs increased the relative abundances of
genera Ensifer, but decreased the relative abundances
of genera Salmonella. Earthworms change the
growing environment of their own gut microbial
communities by swallowing soil containing MPs.
4 CONCLUSIONS
In this work, the changes of earthworms gut bacteria
were observed for a 28-day experiment in the MPs
added soil. The results showed that the microbial
community structure of earthworms gut was
significantly affected by non-biodegradable
polyethylene (PE) and biodegradable poly (butylene
adipate-co-terephthalate) (PBAT) MPs. The number
of operational Taxonomic Units (OTUs) and α
diversity indexes in treatments with MPs were higher
than those in treatment without MPs. The number of
OTUs was in the order of treatments with aged-PE
MPs > treatments with unaged-PE MPs > treatments
with PBAT MPs. The number of OTUs in treatments
with lower MPs concentration was higher than that in
treatments with higher MPs concentration. Addition
of MPs increased the relative abundances of genera
Ensifer, Bacteroides and Bacillus, but decreased the
relative abundances of genera Salmonella,
Escherichia-Shigella and Paracoccus. Therefore, MPs
significantly impact the microbial community of
earthworm gut, which is related to types of MPs.
REFERENCES
BARDGETT R D, VAN DER PUTTEN W H. (2014)
Belowground biodiversity and ecosystem functioning.
Nature, 515(7528):505-511.
BERTRAND M, BAROT S, BLOUIN M, et al. (2015)
Earthworm services for cropping systems. A review.
Agronomy for Sustainable Development, 35(2):553-
567.
GUO X, WANG X, ZHOU X, et al. (2012) Sorption of four
hydrophobic organic compounds by three chemically
distinct polymers: role of chemical and physical
composition. Environmental Science & Technology,
46(13):7252-7259.
HUERTA LWANGA E, GERTSEN H, GOOREN H, et al.
(2016) Microplastics in the Terrestrial Ecosystem:
Implications for Lumbricus terrestris (Oligochaeta,
Lumbricidae). Environmental Science & Technology,
50(5):2685-2691.
HUFFER T, WENIGER A K, HOFMANN T. (2018) Data
on sorption of organic compounds by aged polystyrene
microplastic particles. Data Brief, 18:474-479.
MA L, XIE Y, HAN Z, et al. (2017) Responses of
earthworms and microbial communities in their guts to
Triclosan. Chemosphere, 168:1194-1202.
Effect of Microplastics on Gut Bacterial Community of the Earthworm Pheretima guillelmi
263

SONG Y K, HONG S H, JANG M, et al. (2017) Combined
Effects of UV Exposure Duration and Mechanical
Abrasion on Microplastic Fragmentation by Polymer
Type. Environmental Science & Technology,
51(8):4368-4376.
ZHANG M, JIN B-J, BI Q-F, et al. (2022) Variations of
earthworm gut bacterial community composition and
metabolic functions in coastal upland soil along a 700-
year reclamation chronosequence. Science of The Total
Environment, 804:149-194.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
264
