
Analysis of Possible Ingredient and Manufacture Steps of Oral
GLP-1 Receptor Agonists
Zhiyue Lin
Malvern College Chengdu, Chengdu, 610000, China
Keywords: Oral Glucagon-Like Peptide 1 Receptor Agonists, Excipients, Freeze-Drying, Granulation, Tablet Coating.
Abstract: Glucagon-like peptide 1 receptor agonists (GLP-1RAs) is a efficient medicine treating diabetes 2 and the
dosage form are mainly injection. The Rybelsus is the only oral dosage form at current. This paper analyzes
the possible ingredient and pharmaceutical techniques for different steps when putting GLP-1RAs into
manufacture process like the choose the excipients, granulation and coating. The analysis based on the
research on peptide drugs and the assignment regarding the details of Rybelsus. The data involved come from
different studies or journal and the assignment published by Novo Nordisk. This paper also analyzes the
feature the excipient need to have, the possible advantages and different between the freeze drying, dry
granulation and direct compression and sort out the two with greatest possibility to be applied based on the
feature of API, the importance of coating for biologics will be stated, the function and feature of the coating
will be mentioned and a few common example with good quality will be given.
1 INTRODUCTION
Glucagon-like peptide 1 receptor agonists (GLP-
1RAs) are a member of peptide drug which is a
unique medicine used to treat diabetes 2. At present,
the most common dosage form of GLP-1 receptor
agonists is the solution for injection as the first oral
semaglutide discovered by Novo Nordisk has
finished the clinical trial and being qualified to
publish in 2020 (European Medicines Agency, 2020).
The main difficulties that limit the clinical use of
GLP-1 receptor agonists are the extreme short half-
life time of injection (1.5-5 min) in plasma as it can
be break down by dipeptidyl peptides 4 (DDP-4)
rapidly (Hui, 2002).
According to the statistic of a clinical trial that
have been done in Japan with the purpose to sort out
which dosage form, oral or injection, leads to a better
efficacy and acceptance among Japanese diabetes 2
patients. The overall statistic stated that the oral
dosage form would result in a better blood sugar
control and the risk of hypoglycemia. The oral dosage
form has been provide with a better acceptance
among about 1000 patient (Davies, 2017). This
statistic shows the significance of putting forward the
research on oral GLP-1 receptor agonists for diabetes
2 patient.
This paper will analyze the possible ingredients
and pharmaceutical techniques that could apply to
different manufacture steps of GLP-1RAs tablets
based on the current research progress on GLP-1RAs
and the details of approved sample Rybelsus
(semaglutide).
2 LITERATURE REVIEW
Functionally, glucagon-like peptide 1 receptor
agonists imitate the function of GLP-1 that secret in
to hepatic portal system by the intestinal L cell
located in the colon and distal ileum and this type of
hormone can decrease the concentration of glucagon
and concentration of available fatty acid, slow down
the gastric emptying, increase the insulin sensitivity
and the secretion amount (Baguio, 2007, Hinnen,
2017, Prasad-Reddy, 2015). The other effects of
GLP-1 receptor agonists including positive impacts
on weight, blood pressure and the cholesterol level.
Using GLP-1 receptor agonists to treat diabetes 2 has
a relatively low risk of suffering hypoglycemia as the
effect of GLP-1RAs depend on the blood sugar
concentration and the effect is in direct proportion to
blood sugar level. However, the most common side
effects of taking GLP-1 receptor agonists includes
Lin, Z.
Analysis of Possible Ingredient and Manufacture Steps of Oral GLP-1 Receptor Agonists.
DOI: 10.5220/0011205700003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 321-326
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
321

nausea, vomit and the discomfort at the site of
injection (Prasad-Reddy, 2015).
At present, GLP-1 receptor agonists includes
exenatide, liraglutide, albiglutide, dulaglutide,
lixisenatide and semaglutide. There are great
different between each agent in the pharmacokinetic,
pharmacodynamic and clinics (Prasad-Reddy, 2015).
The most common dosage form of GLP-1 receptor
agonists is the solution for injection as the first oral
semaglutide discovered by Novo Nordisk has
finished the clinical trial and published in 2020
(European Medicines Agency, 2020). The main
difficulties that limit the clinical use of GLP-1
receptor agonists are the extreme short half-life time
of injection (1.5-5 min) in plasma as it can be break
down by dipeptidyl peptides 4 (DDP-4) rapidly (Hui,
2002).
The techniques can apply to the manufacture of
GLP-1 receptor in different steps.
2.1 The Selection of Excipients
The first oral GLP-1 receptor agonist, semaglutide,
which has shown in Figure 1, has been published by
Novo Nordisk in 2020 (European Medicines Agency,
2020). Before Rybelsus has been published, the GLP-
1 receptor agonists can only be used in injection. In
order to designed it into oral dosage form, the
excipients which contribute to the formulation that
Novo Nordisk have chosen are massive. In this
section, the author will use the example of Rybelsus
to analyze what features the excipients should have
so that they can be used to form an oral GLP-1
receptor agonists tablet.
According to the public assignment report that
published by Novo Nordisk, the excipient used in
Rybelsus are salcaprozate sodium, microcrystalline
cellulose, povidone K90 and magnesium stearate
(European Medicines Agency, 2020).
Figure 1: Structural formula of semaglutide.
Microcrystalline cellulose (MCC) is a pure and
partially depolymerized cellulose with the chemical
formulation (C6H10O5)n. It is widely used in
pharmaceutical industry as a disintegrant, filler,
binder and absorbent. It also act as a dry binder and
filler in direct compression as it could improve the
compatibility and compressibility of the mixture.
Overall it is a comprehensive excipients
(Chaerunisaa, 2019). Povidone K90 stands for the
term “polyvinylpyrrolidone K-90”, a soluble PVP
product with the outstanding solubility in all
conventional solvent and it often act as a binder,
bioavailability enhancer and film formation.
Additionally, the ability of Povidone K90 to form
water-soluble complex with active substance to
improve the rate of release and solubility (Folttmann,
2008).
Magnesium stearate, Mg(C
18
H
35
O
2
)
2
, is a
common chemical compound that participate in
formulation as a lubricant to prevent the tablet stick
on the die whereas it increase the liberation and
disintegrate time by acting as a film formation
(Uzunović, 2007).
Salcaprozate sodium (C₁₅H₂₀NNaO₄) is an
excipients that has been mentioned alone in details in
the public assignment report as a new excipient which
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
322
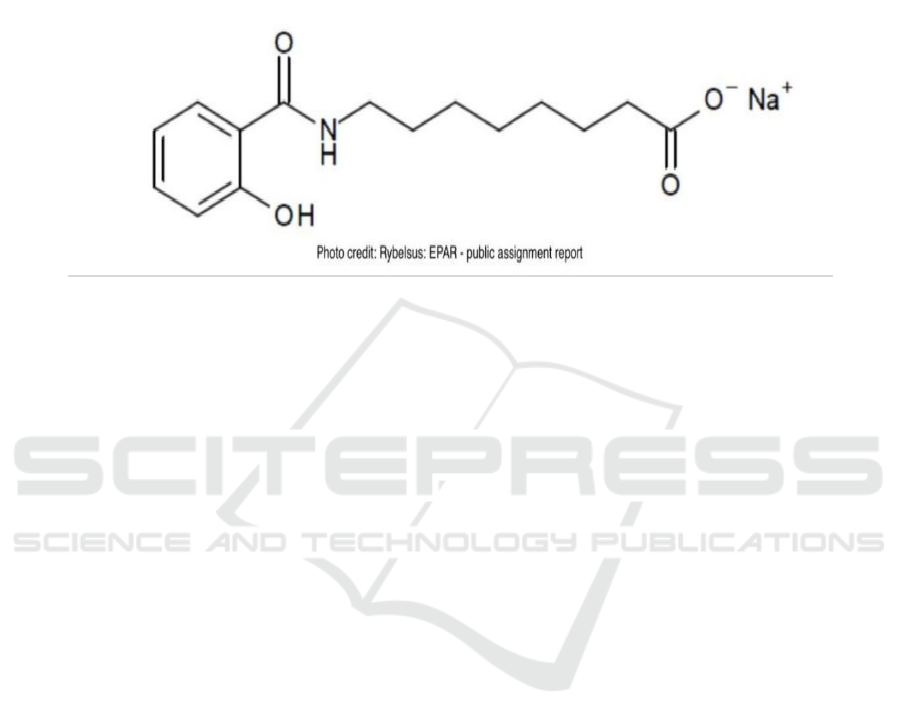
has been added into the formulation to improve the
bioavailability of semaglutide. Basically,
salcaprozate sodium is a sodium salt form of
salcaprozate and it is a powder with the color between
white and almost white, but it worked as an excipient
with polymorphism. As a oral absorption promoter,
the function is to promote the absorption of specific
macromolecules such as insulin. The solubility of
salcaprozate sodium is about 10 mg/ml when the pH
value is between 2-4 but the solubility in the
environment of pH value around 8 is about 300
mg/ml (European Medicines Agency, 2020; Twarog,
2019). The structure is shown in Fig. 2.
Figure 2: Structure of salcaprozate sodium.
In summary, due to the extreme short half-life of
GLP-1 receptor agonists, 3 out of 4 excipients used
have the ability of improving bioavailability and film
formation. Undoubtedly, the general excipients like
filler, disintegrant and binder should be include.
Additionally, the formulation of Rybelsus shows that
most of the excipient should be provided with the
ability to enhance the bioavailability and some
general function.
2.2 Freeze Drying, Granulation and
Direct Compresssion
Both freeze drying and granulation are common in
the manufacture of tablet. However, as a peptide drug
that belong to the biologics, it is more common to
include freeze drying instead of granulation in the
manufacture process due to the similarity between
peptide and protein. Basically, peptide is a short
chain of amino acid that combined by peptide bond,
and the peptide that include over about 50 amino acid
is the big molecule that been named as protein
(Hamley, 2020). The reason of using freeze drying is
that the peptide could denature if it experienced a
high temperature condition so the efficacy of peptide
drug could be reduced (Hamley, 2020). Freeze drying
is a process that dries the peptide drug by place it in
extreme low temperature to turn it into solid state
which is a more stable state because the peptide could
hardly form a chemical reaction such as oxidation
and hydrolysis etc (Rey, 2010).
The process of freeze drying generally involve 3
main steps: 1. Freezing 2. Primary drying or
sublimation of ice 3. Secondary drying of unfrozen
water (Rey, 2010).
In the freezing stage the peptide drug would
usually be put into a condition that the temperature is
about -40°C or even colder for a period of time to
freeze the water completely. The freezing stage is the
most vital part in the whole freeze drying process as
this step would vastly affects the speed on
reconstitution, the duration of freeze drying cycle, the
proper crystallization and the stability of the product.
In the primary drying, the product of last process will
be placed in a vacuum environment and the product
will be heat up to sublimate the ice particles. It is
crucial that the thermal energy has been provided and
the sublimation of ice reach a balance, so the API
could remain active. Some residuary water may
remain in the product, so the goal of secondary drying
is to remove the residuary water but not to dry it
excessively (ensure the appropriate moisture of the
product) (Rey, 2010). Overall, the proper freeze
drying could be a appropriate technique that can
apply to the manufacture of oral GLP-1 receptor
agonists for a few reasons summarised as keeping the
biologics active and ensuring the GLP-1 receptor
agonists would not have adverse reactions. The
drying chamber that participate in the freeze-drying
process has shown in Fig. 3 with its structure (Garcia-
Amezquita, 2016).
Analysis of Possible Ingredient and Manufacture Steps of Oral GLP-1 Receptor Agonists
323
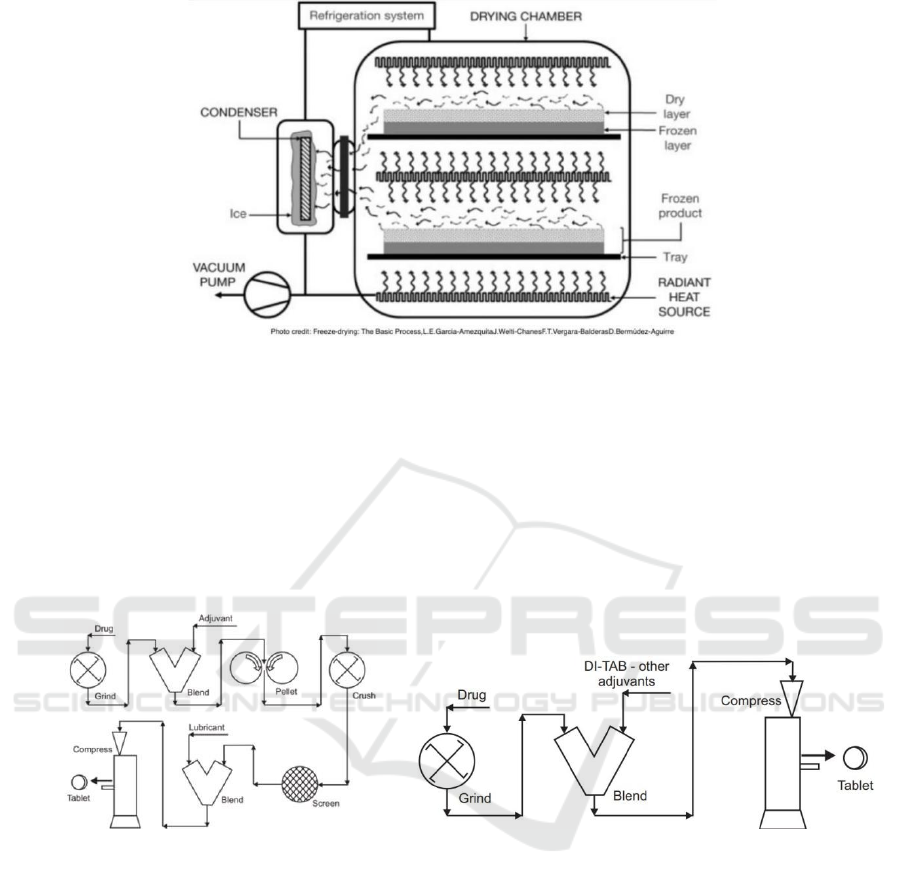
Figure 3: A freeze dryer.
In fact, granulation is a relatively less common
pharmaceutical process that apply to biologics and
peptide drug. However, according to the Novo
Nordisk’s public assignment report, the company
involved the granulation step in the manufacture
process instead of the freeze drying (European
Medicines Agency, 2020), which stated that
granulation can be applied to GLP-1 receptor
agonists as semaglutide falls into the category of
DLP-1 receptor agonists. In the second half part of
this section, the author will analyze the possibility
and appropriateness of different granulation when
applying to the actual production of oral GLP-1
receptor agonists.
The granulation can be divided into 2 types: wet
granulation and dry granulation. Basically, the main
difference is wether the API would be put into a
solvent.
Figure 4: Dry granulation.
Figure 5: Direct compression.
The two main solvent that been used in wet
granulation are aqueous and organic solvent.
However, peptide drug has a low solubility in organic
solvent, the solubility are different from individual
cases. The problem with using aqueous solvent in wet
granulation to manufacture GLP-1 receptor agonists
is the drying step to evaporate the solvent. Generally,
the boiling point of purified water is 100°C, but
most of the peptide and protein will denatured under
such high-temperature condition, so this may be a
risk that the heat cause negative impacts to the API
(GLP-1Ras). As a member of peptide drug family, the
two problem that peptide drug meet when processing
wet granulation would also apply to GLP-1 receptor
(Jenssen, 2008).
Dry granulation is simple and low cost method
and it brings the powder particles together by putting
on a high pressure to the mixture. The general steps
of dry granulation shows in order: grind the API and
excipients to form powders, mix the powder,
compression, screening the product from step 3,
lubricant and disintegrant are added and mixed, final
compression (Jannat, 2016).
Factually, there is two type of compression (step
3) which are slugging process and roller compaction.
The slugging process use tablet press to put on
pressure on the powder particles whereas roller
compaction press the powder to a strip, but these two
steps mainly aimed for the same goal. Even though
the slugging process have the requirement on
compressibility, compression ratio and density of the
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
324

powder (Jannat, 2016), these can be enhanced to
achieve the standard by selecting the appropriate
excipients and design a reasonable formulation. As a
pH and temperature sensitive drug, the whole process
of dry granulation has excluded most of the risky
issue so this may be an possible and ideal technique
that can apply to the manufacture of GLP-1 receptor
agonists in future. The order of the process has shown
in Fig. 4 (Mistry, 2021).
The last technique the author want to mention is
the direct compression (DC), this technique usually
used when then drug is moisture and heat sensitive
(Iqubal, 2014), the later which is the feature that
peptide drugs has been provided with (Garcia-
Amezquita, 2016). In the excipients that Novo
Nordisk used when producing Rybelsus
(semaglutide), microcrystalline cellulose is a material
that has been widely used in the direct compression
stage (Chaerunisaa, 2019), so although the medicine
that can attend direct compression are really limited
as the compressibility and compatibility should be
critically considered, can this technique be applied in
the manufacture of GLP-1 receptor agonists has not
been proved yet. This mostly depend on the
constitution of formulation, which means that the
ingredient powder can form a perfect tablet after
mixed and tablet press, there is no any granulation
included in direct compression (Jannat, 2016).
However, there is still a possibility of involving direct
compression in the manufacture of oral GLP-1
receptor agonists production in future. Each steps of
direct compression are demonstrate in Fig. 5 (Mistry,
2021).
Overall, the freeze drying and dry granulation are
the most appropriate method to manufacture oral
GLP-1 receptor agonists tablet as one is the
traditional and widely accepted manufacture process
for peptide drugs, the latter one is more flexible,
simple and having the advantage of relatively lower
cost if the cost are considered. For the direct
compression, as there is an extreme strict requirement
on the constitution of formulation, the requirement
can be hardly achieved by GLP-1 receptor agonist as
well as other oral medicine.
2.3 Coating
Stomach is a human organ that all oral medicine need
to pass through, the medicine could only reach small
intestine after that. The acidic environment that
between the pH value of 1.5 and 2.5 is mostly
contribute by hydrochloric acid and pepsin, such as
protease which break down proteins are also in the
stomach (Piper,1965). As a member from peptide
family, GLP-1 receptor agonists are also pH sensitive
drugs and it is certain that the drug can easily
denatured and lose the efficacy if the surface does not
covered by any edible anti-acid material.
Tablet coating is a common pharmaceutical
technique with a variety of advantages such as protect
the tablet being broken down by pepsin and provide
a physical and chemical protection to the tablet to
achieve a successful delay release. A polymer-based
film would usually be sprayed on the surface of tablet
after tablet compression or over up the surface of
granules. Additionally, the author could not found
any description on the original taste of the oral GLP-
1 receptor agonists tablet, but the coating could also
be used to enhance the flavor if the taste is not
pleasant (Ankit, 2012).
Take Eudragit as a example, it is a typical sample
of enteric coatings which shows frequently in the
pharmaceutical industry. Eudragit is a poly acrylate
polymers that needs up with different acidic or alkali
end groups, different individuals have their own
solubility in specific ph value as they dissolve by
forming salt with acid/alkali substances. The most
ideal option in Eudragit family seems to be Eudragit
S100, which dissolve under the condition of pH value
greater than 7 and the pH value at the beginning of
the small intestine is about 8, so hopefully the tablet
would break down in the main organ for absorption
and attend a successful delay release (Arruebo,
2020). Additionally, polymer is a chemically inert
compound so the interaction between the coating and
the ingredient could hardly form, which stated that
Eudragit S100 might be a ideal coating material when
manufacturing GLP-1 receptor agonists tablet.
3 CONCLUSIONS
Nowadays, Diabetes has become a common disease
with large group of patient and the age of patient
started to experience a trend of getting younger. In
these cases, the mission of inventing a safer and
effective medicine with a more convenient
administration route become more and more vital and
oral GLP-1 receptor agonist would be an ideal
medicine to put forward the drug therapy for diabetes
2. Manufacturing the GLP-1 receptor agonists into
oral dosage form (tablet) would partially reduce the
inconvenient and the problem with self-injecting
which requires the professional skill. Based on the
approved sample of Rybelsus and the chemical and
biological feature of GLP-1 receptor agonists, the
theoretical analysis shows the possible ingredient and
techniques that may generally apply to most GLP-1
Analysis of Possible Ingredient and Manufacture Steps of Oral GLP-1 Receptor Agonists
325

receptor agonists in key steps when they are put into
manufacture stage. The critical control of variables in
the manufacture process could achieve the best
efficacy of the tablet so that it can be more effective
for diabetes 2 patients. The greatest problem of
putting forward the production of oral tablet is the
short half-life in plasma, however, this problem could
be solved by taking appropriate amount of DDP-4
inhibitor along with GLP-1 receptor agonists but can
not be addressed only by GLP-1RAs.
The analysis may not be specific enough to
theoretically apply to specific peptide drug falls in to
the family because it is based on the general feature
of GLP-1RAs. As Rybelsus is currently the only oral
dosage form, so the excipients sample is relatively
less but still a valuable sample.
ACKNOWLEDGEMENTS
This paper was supported by Pr. Axel Zeitler and Mr.
Wei. this paper would not have been possible without
the academic support provided by Pr. Axel Zeitler
and I would also like to extend my deep gratitude to
Mr. Wei, the teaching assistant who have shown great
understanding to the course and offered a consecutive
help.
REFERENCES
Ankit, G & Ajay, B & Kumar, K M & et al (2012). Tablet
coating techniques: Concepts and recent trends [J]
International Research Journal of Pharmacy, vol.3,
no.9, pp.50-58.
Arruebo, M & Sebastian, V (2020). Chapter 11-Batch and
microfluidic reactors in the synthesis of enteric drug
carriers [M] Nanotechnology for oral drug delivery,
Academic Press.
Baguio, LL & Drucker, DJ (2007). Biology of incretins:
GLP-1 and GIP [J] Gastroenterology, vol.132, no.6,
pp.2131-2157.
Chaerunisaa, AY & Sriwidodo, S & Abdassah, M (2019).
Microcrystalline cellulose as pharmaceutical excipient
[M] Pharmaceutical formulation design-recent
practices.
Davies, M & Pieber, TR & Hartoft-Nielsen, M & Hansen,
OKH & Jabbour, S & Rosenstock, J (2017). Effect of
Oral Semaglutide Compared with Placebo and
Subcutaneous Semaglutide on Glycemic Control in
Patients with Type 2 Diabetes: A Randomized Clinical
Trial [J] The Journal of the American Medical
Association, vol.318, no.15, pp.1460-1470.
European Medicines Agency (2020). Rybelsus: EPAR -
public assignment report, pp. 16–19.
Folttmann, H & Quadir, A (2008). Polyvinylpyrrolidone
(PVP) - one of the most widely used excipients in
pharmaceuticals: An overview [J] International Journal
of Drugs Delivery Technology, vol.8, no.6, pp.22-27.
Garcia-Amezquita, L.E & Welti-Chanes, J & Vergara-
Balderas, F.T & Bermúdez-Aguirre, D (2016).
Encyclopedia of Food and Health,
https://doi.org/10.1016/B978-0-12-384947-2.00328-7
Hamley, I. W (2020), [M] Introduction to peptide science.
Hinnen, D (2017). Glucagon-Like peptide 1 receptor
agonists for type 2 diabetes [J] The Journal of Diabetes
Spectrum, vol.30, no.3, pp. 202-210.
Hui, H & Farilla, L & Merkel, P& Perfetti, R (2002). The
short half-life of glucagon-like peptide-1 in plasma
does not reflect its long-lasting beneficial effects.
European journal of endocrinology [J] Journal of Eur J
Endocrinol, vol.146, no.6, Abstraction.
Iqubal, M K & Singh, P K & Shuaib, M & et al (2014).
Recent advances in direct compression technique for
pharmaceutical tablet formulation [J] International
journal of pharmaceutical research and development,
vol.6, no.1, pp.49-57.
Jannat, E & Arif, A. A & Hasan, M. M & Zarziz, A. B &
Rashid H. A (2016). granulation techniques & its
updated modules [J] The Pharma Innovation Journal,
vol.5, no.10, pp.134-141.
Jenssen, H & Aspmo, S I (2008). [M] Serum stability of
peptides//Peptide-Based drug design, Human Press,
pp.177-186.
Piper, D W & Fenton B H (1965). pH stability and activity
curves of pepsin with special reference to their clinical
importance [J] Gut, vol.6, no.5, pp.506.
Prasad-Reddy, L & Isaacs, D (2015). A clinical review of
GLP-1 RECEPTOR agonists: Efficacy and safety in
diabetes and beyond [J] Drugs In Context, vol.4.
Rey, L & May, J. C (2010). In Freeze drying/lyophilization
of pharmaceutical and biological products, vol.206, pp.
1-85.
S. Mistry (2021), Granulation method, Solution Pharmacy,
https://solutionpharmacy.in/granulation-methods/
Twarog, C & Fattah, S & Heade, J & Maher, S & Fattal, E
& Brayden, D. J (2019). Intestinal permeation
enhancers for oral delivery of macromolecules: A
comparison between Salcaprozate Sodium (SNAC)
and Sodium Caprate (C10) [J] Pharmaceutics, vol.11,
no.2, pp.78-107.
Uzunović, A & Vranić, E (2007). effect of magnesium
stearate concentration on dissolution properties of
ranitidine hydrochloride coated tablets [J] Bosnian
Journal of Basic Medical Sciences, vol.7, no.3, pp. 279-
283.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
326
