
Understanding Allergic Rhinitis and Non-Small Cell Cancer from
Pathology to Treating Practice with Case Reports
Yuxuan Jiang
1,#,* a
, Yu Li
2,# b
, Baiqing Sun
3,# c
and Yongli Zhang
4,* d
1
College of Pharmacy, University of Michigan, Ann Arbor, MI, 48109, U.S.A.
2
Faculty of Art & Science, University of Toronto-St. George, Toronto, ON M5S, Canada
3
Department of Pharmacy, Uppsala University, Uppsala, Uppsala, 75321, Sweden
4
Department of Critical Care Medicine, First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning, China
#
These authors contributed equally to this paper
Keywords: Respiratory Diseases, Allergic Rhinitis, Non-Small Cell Lung Cancer.
Abstract: Recently, COVID-19 has caused a pandemic and received substantial medical attention worldwide.
Subsequently, we recognize the increasing public interest in not only antiviral treatments, but general
respiratory health. Respiratory diseases can take place by endogenous as well as exogenous causes.
Suffering from an unhealthy condition of the respiratory system, from annoying issues as seasonal allergic
rhinitis, all the way to lethal lung cancer brings loads of burden to not only an individual, but also the whole
society. Efficient care and treating practices thus require urgent improvement. Here we present a systematic
study from case reports for the comprehensive understanding of therapeutic options progress focused not
only the currently available, but also under development strategies for the summarised respiratory diseases
based on the pathophysiology understanding. To be specific, currently available treatment options for these
diseases, including pharmacological, immunogenic in according with oncogenic factors and relative
treatments for NSCLC, whose efficacy and side effects are well-characterized. We also discuss and envision
future treatment options that are underway of development, which may lead to advancements in both
potency and reduced adverse effects. Applications of technologies should also be considered promptly by
medical professionals.
1 INTRODUCTION
1
Cells in human bodies need oxygen to stay
functional and alive, inhalation of oxygen from the
atmosphere to the human body depends on the lungs
and the function of the respiratory system. Oxygen
first fills the alveoli and is delivered to each of the
organs through blood vessels. The respiratory
system has numerous functions in addition to gas
exchange, and it is a crucial site where the interior of
our body constantly ‘communicates’ to the extrinsic
environment. People cough and sneeze to protect
their airways from irritants that may cause diseases.
Aerobic organisms have developed numerous
defense mechanisms to protect the airway as they
a
https://orcid.org/0000-0002-2582-6808
b
https://orcid.org/0000-0002-4921-8831
c
https://orcid.org/0000-0001-7966-5627
d
https//orcid.org/0000-0002-4263-8382
evolve. However, respiratory illness remains the
leading cause of death and disability (Soriano 2020).
This main global burden should be thoroughly
studied to enhance the quality of human lives.
Upon understanding the significance of treating
respiratory diseases, we realize that various types of
respiratory diseases that affect different subsections
of the respiratory tract have been investigated in the
medical field. Our review will focus on respiratory
diseases that are highly prevalent and have
contributed heavily to the public health burden, such
as Allergic rhinitis (AR), and Non-Small Cell Lung
Cancer (NSCLC). We selected these diseases as
references for analysis, as they differ in pathogenesis
and treatment options, representing distinct
categories within the broad scope of respiratory
pathology. Until recently, potential therapeutic
targets for all three diseases have been identified.
Several treatment options for AR and NSCLC are
widely implemented in clinical practices, while
416
Jiang, Y., Li, Y., Sun, B. and Zhang, Y.
Understanding Allergic Rhinitis and Non-Small Cell Cancer from Pathology to Treating Practice with Case Reports.
DOI: 10.5220/0011213700003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 416-425
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

optimizations and novel drugs are still being
explored.
In order to assist the public in the understanding
of respiratory diseases, here we provide a systematic
review of allergic rhinitis, COVID-19, and non-
small cell lung cancer from bench to bedsides. We
summarized the pathophysiology of each disease, as
well as research leading to target identification and
currently accepted treatment practices. Furthermore,
we discussed the ongoing research suggesting
potentially novel and more effective treatment
approaches with emphasis on drug development.
This paper is organized in three parts as each talks
about one disease, with an ordered sequence from
the upper to the lower respiratory tract.
2 ALLERGIC RHINITIS
Seasonal allergic rhinitis is caused by oversensitive
immune response to certain allergens in the
environment that are not generally considered
harmful, which mainly affects the upper respiratory
tract. The disease affects a large percentage of the
general population, which leads to a number of
complications that severely impacts patients’ quality
of life and leads to decreased productivity at the
workplace (Lamb 2006). Although allergic rhinitis is
not a particularly serious condition, treatments that
relieve symptoms still constitute a medical need.
The currently accepted therapeutic standards are
antihistamines, intranasal corticosteroids and
antileukotrienes, which target different mediators of
the immune response pathway (Emeryk 2019).
Recently, anticholinergic drugs have received
attention for their anti- inflammatory properties,
which are being repurposed as maintenance therapy
for AR and have gained FDA approval. Some
researchers have also proposed the activation of the
anti-inflammatory cholinergic pathway as a potential
therapeutic approach, which requires further
pharmacological and clinical investigation (Yamada
2018).
2.1 Pathology and Complication
Generally, allergic rhinitis (AR) is characterized by
oversensitive IgE-mediated immune response to
allergens and upper airway inflammation (Wheatley
2015). Symptoms include nasal itching, sneezing,
watery rhinorrhea, and nasal obstruction (Min 2010).
The disease is caused by immune sensitization to a
large variety of inhaled allergens with either indoor
or outdoor origins, including pollen, dust mites, pets,
pests and mold (Wheatley 2015). From the
pathophysiologic perspective, dendritic cells present
characteristic peptide segments on the cell
membrane to form histocompatibility complex
(MHC) class I and II when exposed to allergens,
which signal the transformation of naive CD4+ T
cells to T-helper (Th2) cells. Allergen-specific Th2
cells secrete several Th2-type cytokines such as IL-
3, IL-4, IL-5 and IL-13. Among these cytokines that
are essential for inflammatory responses, IL-4 and
IL-13 promote allergen-specific IgE production by B
cells, which induces the proliferation of eosinophils,
macrophages, and mast cells. Mast cells release
several inflammatory mediators, including histamine
and leukotrienes, which are responsible for increased
nasal itching, vascular permeability, and mucus
hypersecretion (Min 2010, Small 2018, Meltzer
1997). This stage of allergic response constitutes the
early reaction (Min 2010). IL-3 and IL-5, on the
other hand, promotes eosinophil proliferation and
infiltration into the nasal mucosa, which secretes
pro-inflammatory mediators, cationic proteins, and
reactive oxygen species, directly contributing to
increased mucus production and airway constriction
(Antunes 2020). This process triggers the late
reaction, exhibiting nasal congestion, smooth muscle
hyperplasia and airway remodeling as chronic
symptoms (Figure 1) (Min 2010).
Although mild to moderate allergic rhinitis rarely
has serious complications, pharmacological therapy
is recommended by physicians due to the disease’s
significant impacts on patients’ quality of life and
association with secondary inflammatory
complications. AR contributes to unproductive time
at school or work, sleep apnea, and reduced
cognitive abilities, which results in indirect
economic loss comparable to that of mental
disorders and diabetes (Lamb 2006, Wheatley 2015).
Clinical reviews have suggested an increased risk for
several comorbidities if chronic AR is left untreated,
including conjunctivitis, sinusitis, and otitis media
with effusion (OTE) (Min 2010). Study results have
proposed that inflammation involved in AR results
in impaired ciliary function and sinus obstruction,
which creates an anaerobic environment favorable
for bacterial growth that eventually facilitates
middle ear infection and chronic OTE (Bergeron
2005). Other complications of AR, such as nasal
polyposis and adenoid hypertrophy, are suggested by
clinical evidence, although their association with
rhinitis has not been investigated thoroughly (Min
2010).
The association between allergic rhinitis and
asthma is especially pronounced due to their similar
Understanding Allergic Rhinitis and Non-Small Cell Cancer from Pathology to Treating Practice with Case Reports
417

disease pathophysiology. Clinical data has
confirmed that up to 40% of AR patients have
concomitant asthma, and 94% of asthma patients
have AR (Wheatley 2015). In AR patients without
asthma, eosinophil infiltration, lymphocyte number,
and increased IL-5 production in the bronchial
mucosa have been observed after antigen challenge
(Min 2010). It has been proposed that AR-induced
airway hyperresponsiveness contributes to an
increased risk of asthma through migration of IL-5
producing T cells to the bone marrow, which is
associated with an increase in progenitors that can
differentiate into eosinophils (Bergeron 2005).
Eosinophils in the lower airway contribute to airway
remodeling through the release of cytokines and
reactive oxygen species (ROS), which stimulates
mucus hyperproduction, eosinophil recruitment and
damage to the bronchial mucosa (Kudo 2013). Kudo
et al. also pointed out that cytokine and leukotriene
production lead to proliferation of airway smooth
muscle cells and deposition of extracellular matrix
by myofibroblasts (Kudo 2013). The confirmed
association between AR and allergic asthma
becomes the basis for identifying biological targets
that are involved in the pharmacological treatments
of both diseases.
Figure 1: Pathogenesis of allergic rhinitis. Allergen
binding activates the immune system’s intracellular
signaling pathway, releases inflammatory molecules and
causes chemotaxis of immune cells to the upper
respiratory tract tissue (Min 2010).
2.2 Treatments
2.2.1 Current Treatment Options
Several types of pharmacological treatments
available for allergic rhinitis include oral and
intranasal antihistamines, antileukotrienes,
decongestants, and intranasal corticosteroids. These
drugs have well- characterized pharmacokinetic and
pharmacodynamic profiles from clinical trials.
Although each drug class targets a different step in
the inflammatory response pathway, almost all are G
protein-coupled receptor antagonists, demonstrating
the selection of receptor proteins as excellent
therapeutic targets.
Antihistamines target the H1 histamine receptors,
which mainly mediate hypersensitive reactions and
allergic responses (Devillier 2008). At the periphery
level, antihistamines bind to H1 receptors and
stabilize their inactive form, inhibiting allergic
reactions induced by histamine binding (Devillier
2008). Synthesized by lipoxygenase from
arachidonic acid, cysteinyl leukotriene (Cys-LT) is a
class of lipid mediators that act as autocrine and
paracrine factors in eosinophils, which promote the
release of ROS, cationic proteins, and cytokines that
directly contribute to inflammation (Miyata 2020,
Kuehl Frederick 1980). Antileukotrienes inhibit the
effects of Cys-LT as Cys-LT receptor type 1
antagonists, thereby reducing the level of cytokines
and eosinophil chemotaxis to the site of
inflammation (Miyata 2020, Wilson 2004). Note that
dysfunction of arachidonic acid metabolism also
appears in allergic asthma patients, which indicates a
close association between rhinitis and asthma
treatments (Miyata 2020). Corticosteroids are
glucocorticoid receptor antagonists, which are
observed to inhibit the production of multiple
inflammatory mediators, including cytokines (IL-3,
IL-5, granulocyte-macrophage stimulating factor)
and arachidonic acid metabolites. Thus,
corticosteroids suppress T-cell activation, eosinophil
influx, cytokine release, mast cell count, and
histamine content, relieving inflammatory symptoms
effectively (Meltzer 1997). In summary,
antihistamines and antileukotrienes target the final
and intermediate steps, while corticosteroids have an
observable effect on multiple steps of the
inflammatory response pathway.
Among these treatment options, intranasal
corticosteroids have been confirmed as the most
effective by several meta-analyses of randomized,
controlled clinical trials (Wilson 2004). Therefore,
corticosteroids, sometimes in combination with
antihistamines, are recommended by physicians as
the frontline treatment that controls symptoms of
moderate-to-severe allergic rhinitis (Wheatley 2015,
Min 2010, Small 2018). Antihistamines and
antileukotrienes are often prescribed as combined
treatment to control mild persistent allergic rhinitis,
but their potency is less than that of intranasal
corticosteroids (Rodrigo 2006, Ciprandi 2004). We
speculate that the enhanced potency of
corticosteroids can be attributed to its effect on
multiple steps of the inflammatory pathway, which
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
418

is yet to be confirmed by clinical evidence. Due to
the seasonal or perennial nature of allergic rhinitis,
regular administration of symptom-relieving
pharmacological agents during peak season is
usually relevant in the long term for patients with
allergic rhinitis.
In rare cases, allergen immunotherapy may be
applied to desensitize the immune response to
certain allergens systematically (Durham 2016).
Currently available methods include subcutaneous
immunotherapy (SCIT) and sublingual
immunotherapy (SLIT), which are differentiated
based on the routes of delivery (Durham 2016, Cox
2017). SCIT or SLIT requires at least 3 years of
administration, can be costly overall, and can result
in anaphylactic reaction 0.1% of SCIT injection
visits (Cox 2017). Therefore, immunotherapy is
recommended by physicians in limited cases only if
pharmacological therapies are not well-tolerated or
ineffective, which is relevant for only a small
fraction of patients (Wheatley 2015).
2.2.2 Novel Targets in Cholinergic Immune
System
Although corticosteroids are sufficiently potent and
have been the standard allergic rhinitis treatment for
years, recent reviews have discovered that
systematic long-term administration of
corticosteroids is associated with adverse effects on
the cardiovascular, gastrointestinal,
neuropsychiatric, endocrine, and immune systems
(Yasir 2020, Kummer 2006). Therefore, an incentive
was created to develop an alternative drug with
comparable potency. Recently, cholinergic receptor
antagonists, traditionally used as bronchodilators in
the treatment of asthma and chronic obstructive
pulmonary disease (COPD), have received attention
for their anti-inflammatory properties. Although the
use of anticholinergic drugs in allergic rhinitis
treatment is uncommon, several preclinical and
clinical studies have demonstrated their
effectiveness in AR treatment with an improved
safety profile (Pieper 2007, Wessler 2008, Li 2011).
The discovery of anticholinergic drugs as a new
treatment option started with an understanding of the
cholinergic signaling pathway of immune cells.
Traditional views have limited the function of
acetylcholine to the autonomic nervous system, yet
literature has demonstrated solid evidence on the
role of a non-neuronal cholinergic system that
regulates immune function (Verbout 2012).
Experimental results revealed that acetylcholine is
secreted by immune cells and acts as an autocrine
and paracrine factor for airway epithelial and
inflammatory cells (Kistemaker 2012). In the
cholinergic signaling pathway for the inflammatory
response, acetylcholine can be secreted into human
blood, which is transported by organic cation
transporters to the site of infection and signals
chemotaxis of immune cells. Acetylcholine is
detected by muscarinic cholinergic receptors, which
stimulates increased release of cytokines and
chemotactic factors that initiate inflammatory
response. Experiments with human cell lines and
mouse models indicated an effect on lymphocyte
proliferation and cytokine production when known
muscarinic receptor agonists, such as carbanol and
Oxo-M, were applied to stimulate acetylcholine
secretion (Verbout 2012). Collectively, laboratory
evidence supports that muscarinic receptor in this
system are viable drug targets for the attenuation of
an immune response.
Indeed, further research confirmed the
expression of muscarinic receptors (mChA) in
human lymphocytes and characterized the effect of
several muscarinic antagonists, validating M3-
subtype mChA as the target for a novel
immunosuppressant drug. Experimental evidence
suggested the expression of all five subtypes of
muscarinic receptors (M1-M5) in both mouse and
human lymphocytes, including T cells, B cells,
natural killer cells, and macrophages, through
radioligand binding and RT-PCR (Verbout 2012).
Furthermore, studies regarding human airway
epithelial cells confirmed the role of the cholinergic
system in airway remodeling that leads to
bronchoconstriction and mucus production, which
was effectively alleviated by anticholinergic drugs
(Kistemaker 2012, Wang 2019). However, it was
also discovered that there was variance by individual
in the levels of subtype mChA expressed: unlike M1
and M2, M3-M5 receptors are reliably expressed in
all subjects, with M3 being the most abundant
(Tayebati 2002). Therefore, although concerns were
raised regarding the specificity of mChA antagonists
as drugs, it was logical to explore M3 receptor
antagonists as potential drug molecules for
respiratory system inflammation (Jiang 2011). In
fact, several M3 receptor antagonist drugs of the
quaternary ammonium bromide class, such as
tiotropium and ipratropium, have been approved by
the FDA as intranasal sprays that relieve the
symptoms of severe allergic rhinitis (Albertson
2017).
Tiotropium was approved by the FDA in 2004 as
a bronchodilator in maintenance therapy for COPD,
and severe asthma when used in conjunction with
Understanding Allergic Rhinitis and Non-Small Cell Cancer from Pathology to Treating Practice with Case Reports
419
corticosteroids (Albertson 2017). Research has
demonstrated that tiotropium bromide blocks the M3
receptor and regulates apoptosis of immune and
airway epithelial cells, thus inhibiting eosinophil
infiltration and alleviating bronchoconstriction
(Pieper 2007). Recently, bencycloquidium bromide
(BCQB) was approved and became available on the
Chinese market. While tiotropium bromide is
designated for COPD or asthma treatment and
BCQB for allergic rhinitis, their mechanisms of
action are quite similar. Both drugs possess a
quaternary ammonium ion in a six-membered,
bridged ring, a benzene group, and a bromide ion,
indicating similar pharmacokinetic properties and
binding mechanism. To minimize toxicity, these
mChA receptor antagonist drugs were designed as
charged molecules in dosage forms to prevent the
crossing of biological membranes (Albertson 2017).
This similar structure is observed in several short-
acting and long-acting muscarinic receptor
antagonists that were approved by the FDA as
maintenance therapies in COPD and acute asthma.
The recent development of mChA receptor
antagonist drugs has recognized acetylcholine as a
pro- inflammatory signaling molecule, where drugs
were designed to inhibit the chemotactic and
proliferative effects of acetylcholine. However,
some researchers have raised opposing views,
suggesting that ACh released by the central nervous
system might confer anti-inflammatory protective
effects through a distinct neuroimmune pathway
(Grando 2015, Borovikova 2000). Experimental
evidence has proved that low levels of ACh can
inhibit histamine release, as well as activate α-7
nicotinic receptors that cause local anti-
inflammatory effects (Grando 2015). ACh released
by the efferent vagus nerve also reduces the level of
tumor necrosis factor (TNF), a pro-inflammatory
cytokine, and promotes the production of anti-
inflammatory cytokines (Borovikova 2000). Antunes
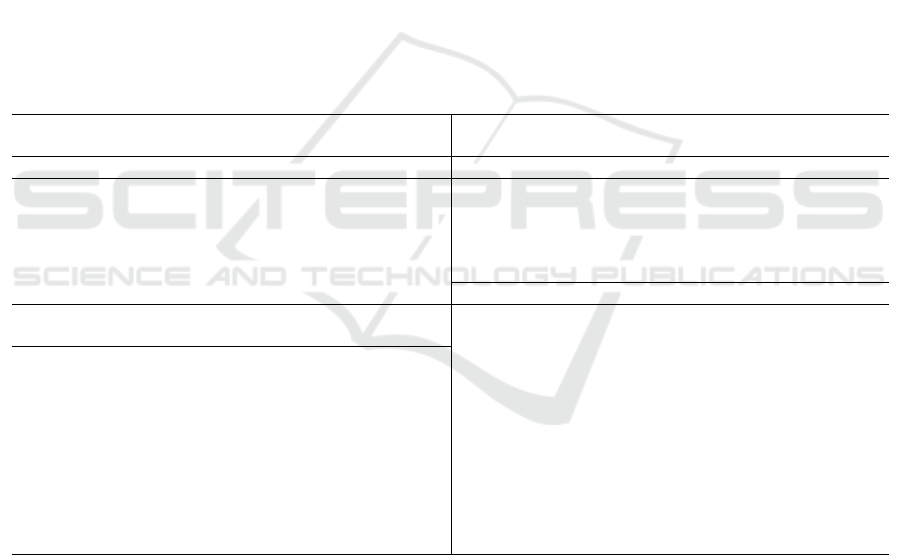
Table 1: Risk factors related to lung cancer (Darby 2005)..
Relative
R
isk factors ris
k
Relative
R
isk factors ris
k
Tobacco use or ex
p
osure Comorbidities
Current smoking 20
Human Immunodeficiency 2 to 11
virus infection
Former smoking 9
Chronic obstructive 2 to 3.1
p
ulmonary disease
Secondhand smoke 1.3 Tuberculosis
—
ex
p
osure Other
Environmental exposures
History of chest radiotherapy 5.9
Famil
y
histor
y
of lun
g
cancer 2
Asbestos 3 Histor
y
of chemothera
py
4.2
Radon 3 Older age
—
Other ex
p
osures
—
Air pollution
Arsenic
Berylliu
m
Beta-carotene in
g
estion
Chromiu
m
Nickel
Soot
et al. also discovered that neostigmine, an
acetylcholinesterase inhibitor, can reduce eosinophil
influx and increase antioxidant defense by
increasing the level of ACh in a BALB/c mice
model (Antunes 2020). Collectively, these studies
provide solid evidence for a cholinergic anti-
inflammatory pathway (CAP), which is worth
further investigation for the future discovery of
viable drug targets.
3 LUNG CANCER AND
NON-SMALL CELL LUNG
CANCER
Non-small cell lung cancer (NSCLC), accounting for
80% to 85% in lung cancer cases, has four main
subtypes which are adenocarcinoma, squamous cell
carcinoma and large cell carcinoma (Liu 2019).
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
420

Though these subtypes start from different lung
cells, they are all classified as NSCLC because of
the similar treatment and prognosis (Ettinger 2017).
Different from small-cell lung cancer (SCLC),
NSCLC tends to grow and spread slower, which is
more likely to be found before spread. For instance,
according to detect mutations in epidermal growth
factor receptor (EGFR), recent trials have suggested
that instead of chemotherapy, initial therapy with
tyrosine kinase inhibitors (TKIs) may be the best
choice for treating NSCLC (da Cunha Santos 2011).
In addition, the use of immune checkpoint inhibitors
(ICIs) has been successfully applied in clinical
cancer treatments (Fan 2017). ICIs targeting
programmed cell death protein 1 (PD-1) and
programmed cell death protein ligand 1 (PD-L1)
display apparent benefits for the treatment of
advanced NSCLC (Xia 2019). We also introduce
other treating options of lung cancer such as
chemotherapy and radiation together with their
perspectives.
3.1 Epidemiology
There are two types of lung cancer, micro-cell lung
cancer, and non-small cell lung cancer (NSCLC)
with the latter accounts for 85% of all lung cancer.
NSCLC tends to be the toxic infection in both
genders (Siegel 2019). Its mortality rate is rather
high when compared with three other most common
occurred cancer types (ie. colon, breast and pancreas
cancer). The life-threatening risk of NSCLC is more
obvious as more than a quarter of patients have died
within 12 months of diagnosis. Besides, the five-
year survival rate is only 17.8% (Kenfield 2008).
Among NSCLC, lamellate cell carcinoma
accounts for 30%. It originates from the first
generation of lamellate cells in the airway epithelial
cells of the lungs. This subdivision is strongly
associated with smoking (Noguchi 1995). About
40% of tumorigenesis happening in the lung is in the
form of adenocarcinoma. Secretory epithelial is
consisted of type II alveolar cells, where are full of
mucus and other nutrients (Gavelli 2000). Morbidity
of adenocarcinoma is the highest among all the
cancer types happened in lung, but unlike lamellate
cell carcinoma, smoking has no obvious effect on its
morbidity (Stellman 1997), so as the age factors.
Results have shown that the use of filters to prevent
smoke from entering the lung had failed but caused
deeper penetration into the lung (Xu 2014). In this
scenario, the smoke becomes carcinogen, and
adenocarcinoma appears. Compared with other
forms of lung cancer, adenocarcinoma grows slowly
and is more likely to be early detected before
metastasis. Large cell cancer accounts for only 5-
10% of lung cancer. There is no obvious symptom
of this type of cancer or tumor growth and as a result
it is usually diagnosed by chance. Bigger cells
carcinomas usually start at the center of the lung and
occasionally causing lymph swellings and limbs
frigi (Whitrow 2003).
Table1 summarized the commonly reported risk
factors of lung cancer. One of the major factors that
cause the lung cancer is smoking. Increasing number
of cigarettes and daily consumption is highly related
with morbidity of lung cancer (Straif 2009). Non-
smokers, however, still have potential risk of
developing lung cancer with risk ratio ranging from
1.14 to 5.20 according to meta-analysis and general
evaluation. The underlying reason is that they live
with smokers (Stayner2008). According to the
American Surgeon General, living with smokers can
increase the risk of non-smoking lung cancer by 20-
30% (Hecht 1999). Radon, a natural cancer-causing
agent, is one of the leading causes of lung cancer,
with an estimated 21,000 deaths from lung cancer in
the United States (Darby 2005). Although Radon has
been in contact with miners sometimes, there is
growing concern that Radon's exposure to natural
gas from uranium deposits are increasing. A series
of randomized controlled trials from North America,
Europe, and China have shown a 2.7-fold increase in
the risk of lung cancer-related lung cancer per liter
(PC).
3.2 Treating Options
Asbestos is used in industry or manufacturing in
conjunction with an improvement in mesothelioma
and lung cancer. The link between asbestos fiber
levels has been found to be a strong indicator of lung
cancer mortality (Wang 2014). As a result, the US
government has implemented steps to decrease the
use of asbestos in marketable and organizational
programs. Additional experimental risk factors
associated with lung cancer comprise the use of
arsenic solvents, disclosure to beryllium and
beryllium oxide; Ingredients include, nickel alloys,
chromium alloys and chloroses ester (Hung 2008).
The disease is more common for the individuals in
the growing periods with effective strategies.
3.2.1 Visual Aided Operation
Patients in stages I, II and III of NSCLCs could have
surgery to remove the tumor if it is feasible for the
tumor as well as the patients. Medical imaging
Understanding Allergic Rhinitis and Non-Small Cell Cancer from Pathology to Treating Practice with Case Reports
421

detection and biopsies help surgeons to distinguish
the originality of tumorigenesis and identify the
patients’ condition in the tumor progression. Video-
enable surgery is now available in clinical practice
and is popular among surgeons. During the surgery,
a tiny camera wrapped in a box is placed in the body
of the patient. Since a large piece of paper does not
need to be cut, a sphere is removed through a piece
of paper [90]. The operation criteria are created on
the basis of achieving a scope that is associated with
a general aim of preventing cancer.
3.2.2 Chemotherapy
Approximately 40% of patients have been diagnosed
in stage IV in recent cases. The goal of treating these
patients is to save their lives and reduce the
incidence of disease-related complications. For the
fourth level NCC, which is Cytotoxic Combination
Chemotherapy, is the first line of treatment that can
affect histology, age and related conditions (PS).
According to the American Society of Clinical
Oncology, patients with 0 or 1 PM are treated with
platinum (cislatine or carbofolatin), pastazal,
gemetitabine, doxtaxil, vinorelbine, erythromycin or
modified. The results of four large randomized
controlled trials have been applied to study platinum
or carboplatin. The results of one of these studies
have shown that the effects of one unit are larger
than the other. The median overall recovery for these
patients was approximately 8-10 months. The
specific combination depends on the type and
frequency of toxic effects and must be determined
individually. However, patients with
adonacarcinoma may benefit from permethrin.
Cysteine is slightly more effective than platinum but
has been proved to induce more side effects. Data
from 2 PPS patients have shown that they only need
one drug, which is not usually platinum. For
chemotherapy, serious events should motivate
agents to change. In addition, if cancer occurs,
therapy should be discontinued when the disease
resolves after four treatment cycles, but the
treatment does not reduce the tumor. 3PS patients do
not routinely use cytotoxic chemotherapy because
the risk of adverse events greatly worsens their
quality of life. More supportive care is generally
recommended for these patients (Hung 2008).
3.2.3 Radiotherapy
Radiotherapy uses the most powerful poles to
damage DNA in cancer cells. This helps to control
or eliminate tumors in the body. Patients with NCCL
who have had chest surgery and are not eligible for
surgery may benefit from radiotherapy.
Radiotherapy may be part of pain relief to improve
the quality of life for patients who do not respond to
surgery or chemotherapy. There are no nearby
lymph nodes for the first NCs with small nodes in
the lungs. Patients are treated with a procedure
called SBRT. This method uses advanced
coordination systems to accurately identify the
tumor and ensure the correct placement of the
tracking device. This allows for stronger and more
focused radiation therapy (Sher 2008). For NCN,
compared with the effectiveness of radiotherapy
with photons, protons, and carbon ions, SBRT
presented a 2-year overall life expectancy, low cost,
and high patient comfort in meta-analysis. In the
next stage study, environmental controls were
significantly higher in patients who did not receive
treatment at SBRT Level 1 NSCC in 70 untreated
patients receiving SBR (Hwang 2003). However,
with NCC, a non-pharmaceutical NC, patients
conducted a three-pronged multidisciplinary study of
SBRT toxicity and efficacy. Of the 55 patients
evaluated, SBRT patients had a 55.8% survival rate
in three years. In these studies, SBRT has been
found to provide surgical treatment for day-to-day
disease- related illnesses to environmental control
and results among certain scope of patients.
3.2.4 Targeting Specific Biomarkers
It helps to improve patient survival by targeting
appropriate molecular targets in private drug tumors
in the NSCLC. Besides, biomarker tests often regard
the quicker and effective way of occupying various
instances needed to maximize the achievement of
the epidermal role. There are agents that have been
successfully targeted in epidermal development
factor receptor (EGFR) mutations and in the
restoration of anaphylactic lymphoma kinase (ALK).
Through genetic testing, ROS1 and RET gene
mutations, MT amplification, and other molecular
changes in B-RAF, HER2, and K-RAS genes may
be targeted for future treatments.
3.2.5 Activating Epidermal Growth Factor
Receptor (EGFR) Gene
When EGFR is activated, it is a cellular tyrosine
kinase receptor that can activate pathways associated
with cell growth and proliferation. This gene carries
to a larger extent the distribution of the factors that
determine the mutation rate of the receptor factor. In
cancer, EGFR mutations continuously trigger
uncontrolled cell division. EGFR gene mutation: 10–
15%
of lung cancer adenocarcinoma of European
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
422
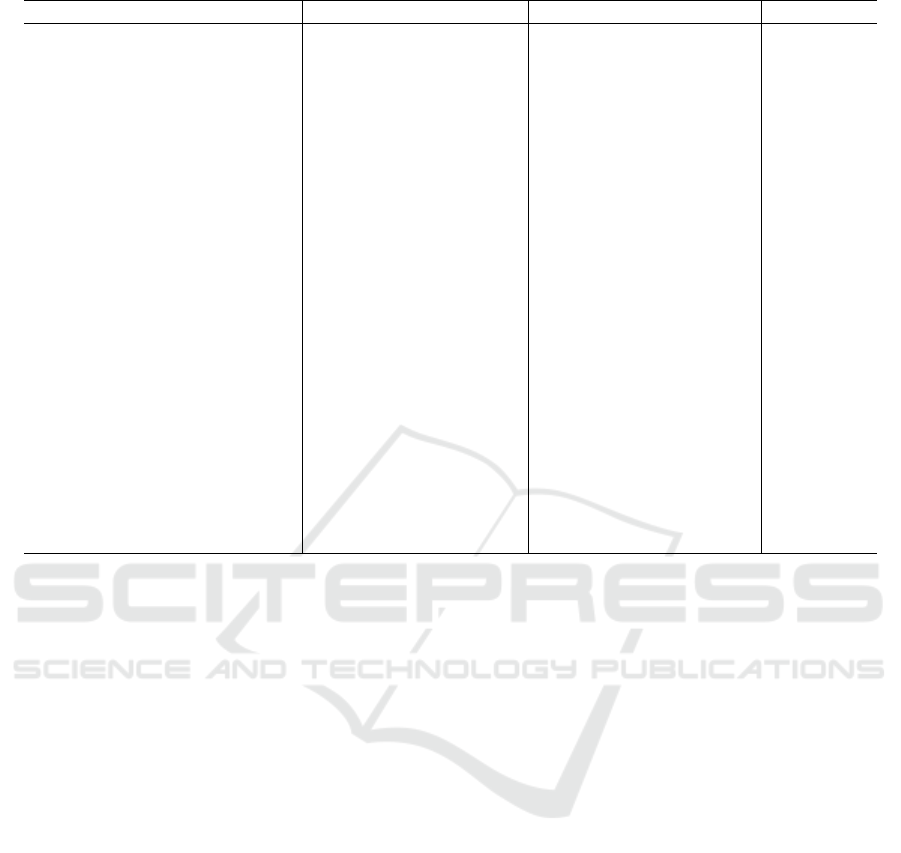
Table 2: Clinical and disease features of patients with EGFR gene (Muscat 1997).
Characteristics Mutation arm (N = 50) Wild-type arm (N = 50) P value
Age, y
0.419
Mean 57.3 59.1
Standard deviation 11.6 10.5
Sex, No. (%)
0.016
Male 18(36.0) 30(60.0)
Female 32(64.0) 20(40.0)
Smoking history, No. (%)
0.043
Smoker 16(32.0) 26(52.0)
Never smoker 34(68.0) 24(48.0)
Histologic type, No. (%)
0.131
ADC 49(98.0) 44(88.0)
SCC 1(2.0) 4(8.0)
Others 0(0) 2(4.0)
Disease stage, No. (%)
0.603
IB 8(16.0) 10(20.0)
IV 42(84.0) 40(80.0)
EGFR-TKI treatment, No.
(%)
ND
No 3(6.0) 26(52.0)
Yes 47(94.0) 24(48.0)
First-line 29(58.0) 5(10.0)
Second-line 15(30.0) 13(26.0)
Third-line or greater
3(6.0) 6(12.0)
and Asian origin, especially in non-smokers and in
women. Although these behaviors are common,
mutation testing is important for patients using
targeted tyrosine kinase inhibitor therapy (Muscat
1997). The risk of exposure to EGFR tyrosine kinase
inhibitors is usually these models encode the EGFR
kinase domain segment. Approximately 90% of
these mutations are due to 19 abrasions and a
mutation of L858R point 21, which corresponds to a
70% response rate for patients receiving erlotinib or
gefitinib treatment.
4 CONCLUSIONS
This paper presents the pathophysiology, current and
prospective treating options for allergic rhinitis (AR)
and non-small cell lung cancer (NSCLC), which
represents certain types of respiratory diseases and
affects distinct regions in the respiratory tract.
Allergic rhinitis is characterized by oversensitive
inflammatory response to an environmental factor
while NSCLC is caused by genetic mutation in the
lung tissue. Medical treatments are available for AR,
from which many target G-protein coupled receptors
and inhibit cell signaling events in the pathogenesis
pathways. With the improved understanding of
pathophysiology, we may find a better option for
treating NSCLC instead of current treatment options
such as operation, chemotherapy, and radiotherapy
to reduce adverse events and improve quality of life
for patients. To enhance the potency and minimize
side effects of treatments, ongoing medical research
on allergic rhinitis and NSCLC have identified novel
therapeutic targets. New drugs differ in action
mechanism as well as novel targets have been
developed and identified. For allergic rhinitis, the
acetylcholine-mediated cholinergic immune system
has been explored for its role in generating
inflammatory response, and several drugs
(tiotropium, ipratropium, bencycloquidium bromide)
targeting muscarinic receptors have been developed
to inhibit the pro- inflammatory effect of
acetylcholine. Future treatments for NSCLC may
emphasize on finding a kinase inhibitor which
functions by blocking a key enzyme or activating
EGFR gene. Recent studies found targeting specific
biomarkers and activating EGFR have been known
as future treatment options of NSCLC. However,
these treatment options may need more future
research to address drug resistance therefore
improve the outcomes in NSCLC patients.
Understanding Allergic Rhinitis and Non-Small Cell Cancer from Pathology to Treating Practice with Case Reports
423

REFERENCES
Albertson, T.E., et al., Muscarinic antagonists in early
stage clinical development for the treatment of asthma.
Expert Opinion on Investigational Drugs, 2017. 26(1):
p. 35-49.
Antunes, G.L., et al., Cholinergic anti-inflammatory
pathway confers airway protection against oxidative
damage and attenuates inflammation in an allergic
asthma model. Journal of Cellular Physiology, 2020.
235(2): p. 1838-1849.
Bergeron, C. and Q. Hamid, Relationship between Asthma
and Rhinitis: Epidemiologic, Pathophysiologic, and
Therapeutic Aspects. Allergy, Asthma & Clinical
Immunology, 2005. 1(2): p. 81.
Borovikova, L.V., et al., Vagus nerve stimulation
attenuates the systemic inflammatory response to
endotoxin. Nature, 2000. 405(6785): p. 458-462.
Ciprandi, G., et al., Antihistamines added to an
antileukotriene in treating seasonal allergic rhinitis:
histamine and leukotriene antagonism. Eur Ann
Allergy Clin Immunol, 2004. 36(2): p. 67-70, 72.
Cox, L.S., Sublingual Immunotherapy for Allergic
Rhinitis: Is 2-Year Treatment Sufficient for Long-term
Benefit? JAMA, 2017. 317(6): p. 591-593.
da Cunha Santos, G., F.A. Shepherd, and M.S. Tsao,
EGFR Mutations and Lung Cancer. Annual Review of
Pathology: Mechanisms of Disease, 2011. 6(1): p. 49-
69.
Darby, S., et al., Radon in homes and risk of lung cancer:
collaborative analysis of individual data from 13
European case-control studies. BMJ, 2005. 330(7485):
p. 223.
Devillier, P., N. Roche, and C. Faisy, Clinical
Pharmacokinetics and Pharmacodynamics of
Desloratadine, Fexofenadine and Levocetirizine.
Clinical Pharmacokinetics, 2008. 47(4): p. 217- 230.
Durham, S.R. and M. Penagos, Sublingual or
subcutaneous immunotherapy for allergic rhinitis?
Journal of Allergy and Clinical Immunology, 2016.
137(2): p. 339-349.e10.
Emeryk, A., J. Emeryk-Maksymiuk, and K. Janeczek,
New guidelines for the treatment of seasonal allergic
rhinitis. Advances in Dermatology and
Allergology/Postępy Dermatologii i Alergologii, 2019.
36(3): p. 255-260.
Ettinger, D.S., et al., Non-Small Cell Lung Cancer,
Version 5.2017, NCCN Clinical Practice Guidelines in
Oncology. J Natl Compr Canc Netw, 2017. 15(4): p.
504-535.
Fan, Y. and W. Mao, Immune checkpoint inhibitors in
lung cancer: current status and future directions.
Chinese Clinical Oncology; Vol 6, No 2 (April 2017):
Chinese Clinical Oncology, 2017.
Gavelli, G. and E. Giampalma, Sensitivity and specificity
of chest x-ray screening for lung cancer. Cancer, 2000.
89(S11): p. 2453-2456.
Grando, S.A., et al., Recent progress in revealing the
biological and medical significance of the non-
neuronal cholinergic system. International
Immunopharmacology, 2015. 29(1): p. 1-7.
Hecht, S.S., Tobacco Smoke Carcinogens and Lung
Cancer. JNCI: Journal of the National Cancer Institute,
1999. 91(14): p. 1194-1210.
Hung, R.J., et al., A susceptibility locus for lung cancer
maps to nicotinic acetylcholine receptor subunit genes
on 15q25. Nature, 2008. 452(7187): p. 633-637.
Hwang, S.-J., et al., Lung cancer risk in germline p53
mutation carriers: association between an inherited
cancer predisposition, cigarette smoking, and cancer
risk. Human Genetics, 2003. 113(3): p. 238-243.
Jiang, J.-X., et al., Characterization of bencycloquidium
bromide, a novel muscarinic M3 receptor antagonist in
guinea pig airways. European Journal of
Pharmacology, 2011. 655(1): p. 74-82.
Kenfield, S.A., et al., Comparison of aspects of smoking
among the four histological types of lung cancer.
Tobacco Control, 2008. 17(3): p. 198.
Kistemaker, L.E.M., et al., Regulation of airway
inflammation and remodeling by muscarinic receptors:
Perspectives on anticholinergic therapy in asthma and
COPD. Life Sciences, 2012. 91(21): p. 1126-1133.
Kudo, M., Y. Ishigatsubo, and I. Aoki, Pathology of
asthma. Frontiers in Microbiology, 2013. 4: p. 263.
Kuehl Frederick, A. and W. Egan Robert, Prostaglandins,
Arachidonic Acid, and Inflammation. Science, 1980.
210(4473): p. 978-984.
Kummer, W., et al., Role of acetylcholine and polyspecific
cation transporters in serotonin- induced
bronchoconstriction in the mouse. Respiratory
Research, 2006. 7(1): p. 65.
Lamb, C.E., et al., Economic impact of workplace
productivity losses due to allergic rhinitis compared
with select medical conditions in the United States
from an employer perspective. Current Medical
Research and Opinion, 2006. 22(6): p. 1203-1210.
Li, J., et al., Subchronic toxicity and toxicokinetics of
long-term intranasal administration of
bencycloquidium bromide: A 91-day study in dogs.
Regulatory Toxicology and Pharmacology, 2011.
59(2): p. 343-352.
Liu, J.-C., et al., A Review on the Antitumor Activity of
Various Nitrogenous-based Heterocyclic Compounds
as NSCLC Inhibitors. Mini-Reviews in Medicinal
Chemistry, 2019. 19(18): p. 1517- 1530.
Meltzer, E.O., The pharmacological basis for the treatment
of perennial allergic rhinitis and non- allergic rhinitis
with topical corticosteroids. Allergy, 1997. 52(s36): p.
33-40.
Min, Y.-G., The Pathophysiology, Diagnosis and
Treatment of Allergic Rhinitis. Allergy Asthma
Immunol Res, 2010. 2(2): p. 65-76.
Miyata, J., et al., Cysteinyl leukotriene metabolism of
human eosinophils in allergic disease. Allergology
International, 2020. 69(1): p. 28-34.
Muscat, J.E., et al., Cigarette smoking and large cell
carcinoma of the lung. Cancer Epidemiology
Biomarkers & Prevention, 1997. 6(7): p.
477.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
424

Noguchi, M., et al., Small adenocarcinoma of the lung.
Histologic characteristics and prognosis. Cancer,
1995. 75(12): p. 2844-2852.
Pieper, M.P., N.I. Chaudhary, and J.E. Park,
Acetylcholine-induced proliferation of fibroblasts and
myofibroblasts in vitro is inhibited by tiotropium
bromide. Life Sciences, 2007. 80(24): p. 2270-2273.
Rodrigo, G.J. and A. Yañez, The role of antileukotriene
therapy in seasonal allergic rhinitis: a systematic
review of randomized trials. Annals of Allergy,
Asthma & Immunology, 2006. 96(6): p. 779-786.
Sher, T., G.K. Dy, and A.A. Adjei, Small Cell Lung
Cancer. Mayo Clinic Proceedings, 2008. 83(3): p. 355-
367.
Siegel, R.L., K.D. Miller, and A. Jemal, Cancer statistics,
2019. CA: A Cancer Journal for Clinicians, 2019.
69(1): p. 7-34.
Small, P., P.K. Keith, and H. Kim, Allergic rhinitis.
Allergy, Asthma & Clinical Immunology, 2018. 14(2):
p. 51.
Soriano, J.B., et al., Prevalence and attributable health
burden of chronic respiratory diseases,
1990–2017: a systematic analysis for the
Global Burden of Disease Study 2017. The Lancet
Respiratory Medicine, 2020. 8(6): p. 585-596.
Stellman, S.D., et al., Impact of Filter Cigarette Smoking
on Lung Cancer Histology. Preventive Medicine,
1997. 26(4): p. 451-456.
Stayner, L., et al., An epidemiological study of the role of
chrysotile asbestos fibre dimensions in determining
respiratory disease risk in exposed workers.
Occupational and Environmental Medicine, 2008.
65(9): p. 613.
Straif, K., et al., A review of human carcinogens—
Part C: metals, arsenic, dusts, and fibres. The Lancet
Oncology, 2009. 10(5): p. 453-454.
Tayebati, S.K., et al., Muscarinic cholinergic receptor
subtypes in the hippocampus of aged rats.
Mechanisms of Ageing and Development, 2002.
123(5): p. 521-528.
Verbout, N.G. and D.B. Jacoby, Muscarinic Receptor
Agonists and Antagonists: Effects on Inflammation
and Immunity, in Muscarinic Receptors, A.D. Fryer,
A. Christopoulos, and N.M. Nathanson, Editors. 2012,
Springer Berlin Heidelberg: Berlin, Heidelberg. p.
403-427.
Wang, J., et al., Effect of Tiotropium Bromide on Airway
Inflammation and Programmed Cell Death 5 in a
Mouse Model of Ovalbumin-Induced Allergic
Asthma. Canadian Respiratory Journal, 2019. 2019: p.
6462171.
Wang, Y., et al., Rare variants of large effect in BRCA2
and CHEK2 affect risk of lung cancer. Nature
Genetics, 2014. 46(7): p. 736-741.
Wessler, I. and C.J. Kirkpatrick, Acetylcholine beyond
neurons: the non-neuronal cholinergic system in
humans. British Journal of Pharmacology, 2008.
154(8): p. 1558-1571.
Wheatley, L.M. and A. Togias, Allergic Rhinitis. New
England Journal of Medicine, 2015. 372(5): p. 456-
463.
Whitrow, M.J., et al., Environmental exposure to
carcinogens causing lung cancer: Epidemiological
evidence from the medical literature. Respirology,
2003. 8(4): p. 513-521.
Wilson, A.M., P.M. O'Byrne, and K. Parameswaran,
Leukotriene receptor antagonists for allergic rhinitis: a
systematic review and meta-analysis. The American
Journal of Medicine, 2004. 116(5): p. 338-344.
Xia, L., Y. Liu, and Y. Wang, PD-1/PD-L1 Blockade
Therapy in Advanced Non-Small-Cell Lung Cancer:
Current Status and Future Directions. The Oncologist,
2019. 24(S1): p. S31-S41.
Xu, B., R. Chetty, and B. Perez-Ordoñez, Neuroendocrine
Neoplasms of the Head and Neck: Some Suggestions
for the New WHO Classification of Head and Neck
Tumors. Head and Neck Pathology, 2014. 8(1): p. 24-
32.
Yamada, M. and M. Ichinose, The cholinergic anti-
inflammatory pathway: an innovative treatment
strategy for respiratory diseases and their
comorbidities. Current Opinion in Pharmacology,
2018. 40: p. 18-25.
Yasir, M., et al., Corticosteroid Adverse Effects. 2020:
StatPearls Publishing, Treasure Island (FL).
Understanding Allergic Rhinitis and Non-Small Cell Cancer from Pathology to Treating Practice with Case Reports
425
