Analysis of the Influence of Nitrite for Public Health
Kang Qiao
Qingdao NO.2 Middle School, Qingdao, 266100, China
Keywords: Nitrite, Public Health, Cardiovascular Disease, Nitrite Pollution.
Abstract: Nowadays, it is necessary to study whether nitrite should be limited strictly or used normally, since nitrite has
both benefits and probable harms. Researchers hold different opinions of nitrite, including if nitrite should be
ingested by people, making people confused on who to believe and which instruction to follow. Thus, the
author conducts this study to have an overview on the previous literature and analyzes the related information
in order to give suggestions to people. Through the analysis, it is concluded that nitrite has advantages of
curing cardiovascular disease, and defensing bacteria. Additionally, it has controversial harms of causing
cancer and deformity, as well as the doubtless harm of nitrite poisoning. To deal with the benefits and harms
of nitrite, people should ingest nitrite properly and the government need to take actions to reduce nitrite
pollution.
1 INTRODUCTION
Nitrite commonly forms in daily food, especially
vegetables, barbecue, and pickled food. With the
development of technology and the improvement of
living standard, people have progressively paid more
attention to food safety, so nitrite in food becomes one
of the hot spots. Nowadays, it is controversial that if
nitrite should be limited strictly or used normally
since nitrite is both beneficial in some aspects, and
has some uncertain harms, such as causing cancer and
deformity. To be specific, news and some articles
provided by social media tell people to focus on the
carcinogenicity and teratogenicity of nitrite, and
provide advice, such as warning people not to drink
water that has been boiled for several times, which
contains more nitrite than normal water. What is
more, there are lots of studies and experiments to
prove that nitrite does not cause cancer and deformity,
but has plenty of benefits. Under this circumstance,
people hesitate about which opinion to believe so that
they may not have the proper strategy to control
nitrite. Therefore, it is necessary to review the
positive and negative influences of nitrite, and give
some suggestions on how to deal with nitrite when we
have not solved this controversy. This paper would
elaborate these points to help people understand
nitrite more clearly, and tell individuals as well as
departments of government to control nitrite to
maximize the benefit and minimize the probable
harms.
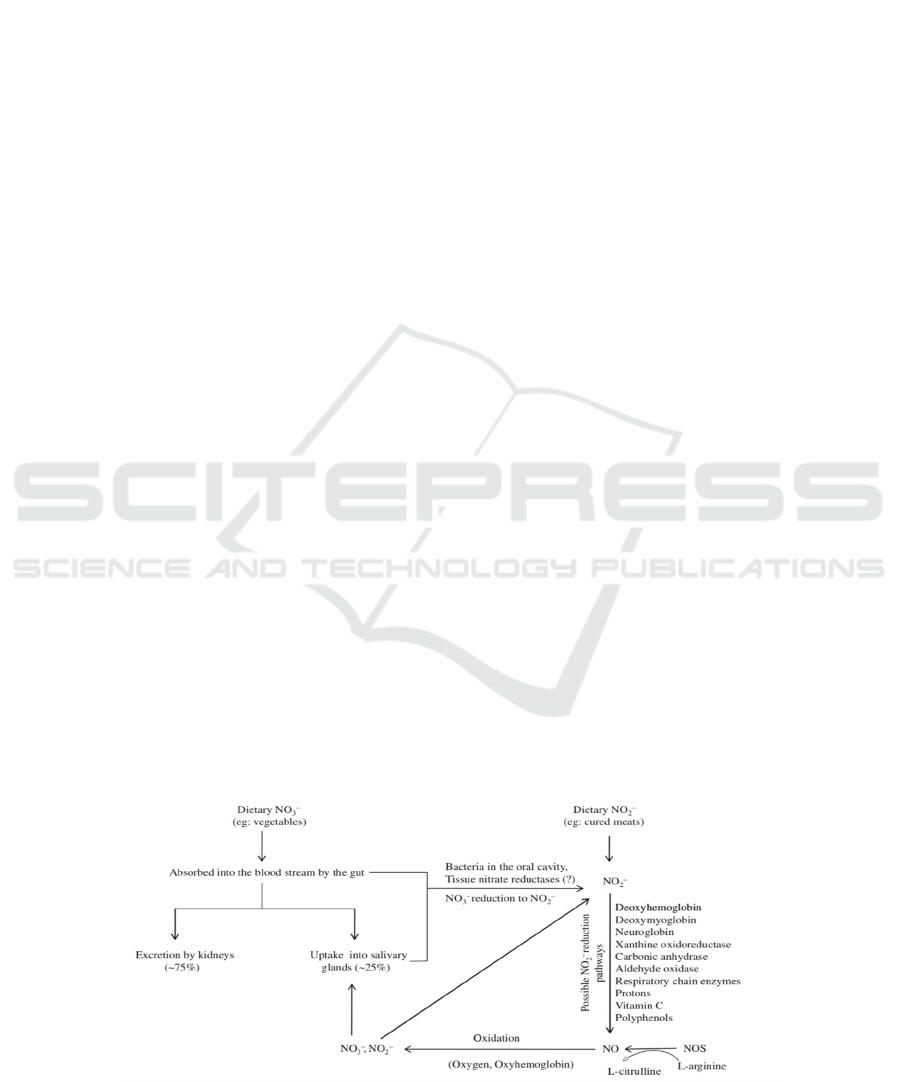
Figure 1: Schematic presentation of nitric oxide (NO) generation and metabolism in the body (Machha, Schechter 2011).
200
Qiao, K.
Analysis of the Influence of Nitrite for Public Health.
DOI: 10.5220/0011243100003438
In Proceedings of the 1st International Conference on Health Big Data and Intelligent Healthcare (ICHIH 2022), pages 200-205
ISBN: 978-989-758-596-8
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
2 ANALYSIS OF THE BENEFITS
AND HARMS OF NITRITE
2.1 Benefits of Nitrite
2.1.1 Management of Cardiovascular
Disease
As can be seen in figure 1, nitrite can be reduced
to be NO in human body. Up till now, scientists have
found many ways to reduce nitrite to be NO. For
example, nitrite can be reduced by
deoxyhemoglobin, deoxymyoglobin, xanthine
oxidoreductase, cytochrome P450 enzymes,
mitochondrial respiratory chain enzymes, aldehyde
oxidase, carbonic anhydrase, acidic
disproportionation, and reducing agents (e.g.
ascorbate, polyphenols), which only have high
activity in the low-PH and low-oxygen condition
(Machha, Schechter 2011). Additionally, arterial
venous gradient of nitrite in human forearm
circulation shows that nitrite is metabolized to NO
across physiological pH and oxygen level (Cosby,
Partovi, et al. 2003). Thus, NO, reduced from nitrite
and can be formed in low-oxygen condition, is the
substitution of endothelium-derived NO, which is
synthesized from the amino acid L-arginine by the
eNOS enzyme with oxygen. Thus, there would be
more NO in cardiovascular system if people ingest
nitrite.
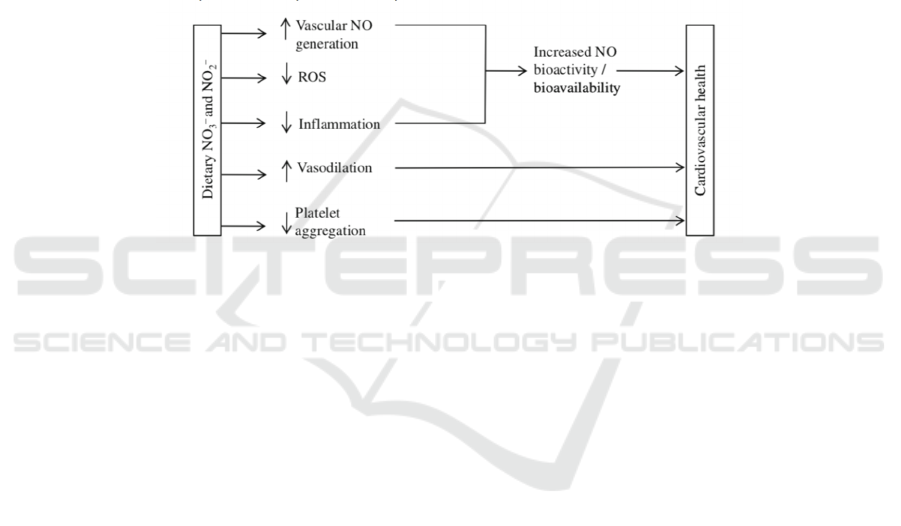
Figure 2: Schematic presentation of potential mechanisms by which dietary nitrite and nitrate could modulate cardiovascular
health (Machha, Schechter 2011).
NO plays a significant role in improving the
endothelial function since NO can help to maintain
the vascular homeostasis. To be specific, as can be
seen in figure 2, NO can control smooth muscle cells
and circulating blood cells to manage the
proliferation of smooth muscle cell, activity of
platelet, and vasodilation of blood vessels (Naseem
2005). Hence, the lack of NO would cause endothelial
dysfunction, leading to the disruption of vascular
homeostasis, and then result in many cardiovascular
problems. On the contrary, sufficient amount of nitrite
would make cardiovascular diseases below to be
cured.
As for thrombus, abnormal aggression of platelet
would lead to thrombus, which would contribute to
the cerebral thrombosis, myocardial infarction, or
ASO. Fortunately, the intake of nitrite can inhibit the
aggregation of platelet (Johnson et al. 1990). As for
Myocardial ischemia, reperfusion therapy is one of
the most effective treatment to decrease the size of
myocardial infarction area by making blood go back
to the heart. However, reperfusion therapy would
cause myocardial damage, which is a very serious
problem, leading to heart failure, cardiac fibrosis, and
even sudden death. Recent research display that
nitrite would reduce the damage to heart. For
example, Webb et al. found that the retrograde
perfusion with nitrite can decrease the myocardial
infarction area by 60% during the treatment of
retrograde perfusion (Webb et al. 2004).
Hypertension has been one of the commonest disease
in cardiovascular diseases. Nowadays, plenty of
people, especially the middle-aged and the elderly,
are suffered or even die because of hypertension. As
an important way to cure hypertension, the treatment
of nitrite works out pretty well. Scientists found that
18.8 mg of nitrate per kilogram body weight per day,
which would be transformed to nitrite inside body
(Feng 1983), would decrease diastolic blood pressure
by 4.5 mm (Hg Sobko et al. 2010).
2.1.2 Defense against Bacteria
Nitrite can inhibit the growth of anaerobic bacteria,
such as Achromobacter, Aerobacter, Escherichia,
Flavobacterium, Micrococcus, and Pseudomonas.
The function of nitrite of defensing against bacteria,
in my opinion, can be divided into three parts—oral
cavity, gastrointestinal tract, and skin. As for oral
cavity, 25% of the intake of nitrate into human body
Analysis of the Influence of Nitrite for Public Health
201

would be secrete to oral cavity, and then be reduced
to nitrite by symbiotic bacteria in mouth. Because of
the antibacterial function of nitrite, many bacteria in
mouth can be inhibited. As for gastrointestinal tract,
acidic environment provided by gastric acid would
acidify nitrite, helping to kill bacteria. To illustrate,
Dykhuizen et al. conducted experiment that make
Yersinia enterocolitica, Salmonella Enteritidis,
Shigella sonnei, and Escherichia coli O157 to be
exposed different concentration of nitrite and
different PH. As a result, they found that the acidic
condition and nitrite would cooperate to kill pathogen
(Dykhuizen et al. 1996). Skin is also a weak acidic
environment because nitrite would be released to the
surface of skin with sweat, and nitrite can play a role
in defensing bacteria on skin.
2.2 Harms of Nitrite
2.2.1 Carcinogenicity of Nitrite
The carcinogenicity of nitrite is still sort of
controversial. Some scientists are big supporters of
the opinion that nitrite would lead to cancer. First, the
mechanism of nitrite is very clear. Nitrite does not
cause cancer directly, and most of the intake of nitrite
would be excreted out of body along with urine.
Nevertheless, under acidic condition (PH 1-4) some
of nitrite would decompose into nitrous acid, which
would then decompose into nitroso due to the
instability. After this, nitroso would combine with
secondary amine, a kind of metabolites of protein, to
synthesize nitrosamine, which is a sort of very strong
carcinogen. Nitrosamine would methylate guanine of
RNA and DNA to make mutation occur in cells
(Zhang et al. 2015). Second, some experiments, such
as the experiment conducted by U.S. National
Toxicology Program, showed that although there was
no evidence showing that the sodium nitrite had
carcinogenic activity in the group of female F344/N
rats and male B6C3F1 mice, they found that maybe
the carcinogenic activity displayed in the group of
female B6C3F1 mice according to the positive trend
in the incidences of squamous cell papilloma or
carcinoma (combined) in the forestomach (Program
2008).
Other scientists believe that nitrite does not have
the property of carcinogenicity, so they contradicts
the opinion above respectively. First, the formation of
great amount of nitrosamine is not convincing.
Specifically, nitrite requires nitrite reductase from
certain bacteria to catalyze the reaction of synthesis
of nitrosamine, but healthy people have very little this
sort of bacteria in their stomach. What is more, 500
mg vitamin C can reduce the formation of
nitrosamine in stomach reduce by 99%, so the
everyday diet would almost prevent the formation of
it. What is more, attributing to the acidic environment
in stomach, nitrite would be quickly reduced into NO,
and then released out of body (Griesenbeck et al.
2009). Therefore, it is no need to worry about the
nitrosamine transformed from nitrite. Second, the
experiment of rats and mice is not convincing either.
This research only offer the evidence of
carcinogenicity in forestomach of female B6C3F1
mice, but not offer evidence of carcinogenicity in
other organs or tissues in both female and male mice
and rats (Bryan et al. 2012). Third, as evidenced by
information in figure 3 shown below (Bryan et al.
2012), scientist indicates that the a majority of
research proving that nitrite is carcinogenicity was
conducted several decades ago, and most of more
recent, and better-designed studies showed that nitrite
was not related to cancer.
However, some other scientists raises new point
that nitrite does not cause cancer, but induce cancer
(Huang et al. 2009). Specifically, before the
formation of tumour, cancer cells has already attain
denitrification gene from some symbiotic bacteria
with anaerobic metabolism in human body, and then
survive as cancer stem cells. In certain condition,
these cancer stem cells would move to tumour, and
the gene with the function of denitrification would be
activated. These cells would choose nitrite respiration
to adapt to the microenvironment surrounding the
tumour. Hence, as the source of nutrient of cancer
cells, nitrite may promote the growth of them, but not
cause cancer. However, this idea does not have
enough data, or material supporting it so that the truth
of this theory is doubted.
2.2.2 Teratogenicity of Nitrite
Like the carcinogenicity, the teratogenicity of nitrite
is also controversial. Many materials show the
teratogenicity of nitrite. For example, NaNO2 would
damage the DNA of supporting cell of testis when the
dose of NaNO2 is larger than 150 μg /ml (Ren 2007).
Some other information disproves the teratogenicity
of nitrite. For instance, 0-100 mol/L sodium nitrite
under neutral condition would not hurt DNA of
epithelial cells in stomach. Only when PH falls to 4.2
and the dose of sodium nitrite is more than 50
mmol/L, the DNA might be damaged (Smith et al.
2006). Thus, people cannot reach that strict standard
of environment in their daily life, so the harm
stemming from the teratogenicity would not occur.
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
202

Figure 3: Animal Toxicological Studies of Nitrite Carcinogenicity with Serious Methodological Limitations (Bryan et al.
2012).
2.2.3 Nitrite Poisoning
Nitrate poisoning, also called enterogenous cyanosis,
resulted from too much intake of nitrite or nitrate (a
great part of nitrate would be reduced into nitrite in
human body). 0.3-0.5g nitrite would cause nitrite
poisoning, and more than 3g nitrite would cause death
(Ye 2007). After nitrate gets into human’s body, it
would react with ferrous ion in hemoglobin to oxidize
normal hemoglobin into hemoglobin, which does not
have the ability of carrying oxygen (Ye 2007). In this
regard, all the tissues and organs in human body
would confront oxygen deficit, leading to the
symptoms mentioned above.
3 MEASURES OF
CONTROLLING NITRITE
According to the benefits and harms of nitrite above,
it is difficult to completely prohibit the use of nitrite
because of its benefits in treatment and prevention of
cardiovascular diseases, and the function of defensing
bacterial. Moreover, we cannot intake nitrite as much
as we want since scientists are not sure if nitrite has
car and tera properties, and too much nitrite would
lead to poisoning. Thus, some instructions or
measures are needed to avoid the possible harms and
attain benefits.
3.1 Distinguishing Susceptible
Population
Given the probable harms of nitrite because of the
doubted carcinogenicity and teratogenicity, it is
necessary to find susceptible population, and develop
special diet standard for them to avoid these harms
(Huang et al. 2009). Susceptible population here
might include ones who have medical history of
cancer, especially gastric cancer, in family, ones who
have serious stomach problems, and so on. As for
these susceptible population, they should follow their
own diet standards, such as avoiding consuming
barbecue and pickled food, which contain relatively
high amount of nitrite. If possible, they should also
remember to blanch some vegetables high in nitrite
before cooking, like spinage, to reduce nitrite.
Analysis of the Influence of Nitrite for Public Health
203

3.2 Avoiding Excessive Intake of Nitrite
Too much intake of nitrite would lead to nitrite
poisoning, so all of people should pay attention to the
amount of nitrite they ingest. For instance, people
should not purchase pickled food without having
labels of expiration rate, and ingredient list, in
informal stalls. According to the analysis of test result
of nitrite content in cooked meat product in Zhoukou
in 2014-2015, products with nitrite beyond the
standard have a proportion of 34.87% among all the
products, and the maximum nitrite content is
1370mg/kg (Zhang, Lu, Sun 2017). If people
consume plenty of packaged food like these, they
might be suffered from nitrite poisoning.
3.3 Control of Nitrate Pollution in
Vegetables
In most of areas in China, nitrates (which would be
reduced into nitrite) in several kinds of leafy and root
vegetables with relatively high consumption are
much higher than normal, and the one with highest
content is 9 times as standard (Ye 2007). Under this
circumstance, it is necessary for the governments and
organizations to control nitrate pollution in
vegetables. Here are some advice: Governments can
request farmers to select vegetables species which
cannot accelerate much nitrate; the Bureau of
Agriculture can come up with new way to use
fertilizers, such as coordinating nitrogen fertilizers
with organic fertilizers.
3.4 Dietary Intervention of Nitrite
Nitrite has the ability of curing cardiovascular
diseases, so doctors can design special daily diet plans
for patients who suffer from cardiovascular diseases,
especially hypertension. According to different
conditions, doctors can adjust the intake of nitrite for
patients in everyday meals.
4 DISCUSSION
This paper analyzes both the benefits and the
probable harms of nitrite comprehensively, and
provide some measures to control the use of nitrite to
not only prevent people from harms, but also take
advantage of nitrite. According to the evidence
provided by past papers, the opinion that nitrite does
not have carcinogenicity and teratogenicity is more
convincing because of the clear, logical arguments,
and plenty of accurate date from experiments.
Nevertheless, the evidence supporting the
carcinogenicity or teratogenicity of nitrite is sort of
insufficient, and has some holes. Although perhaps
nitrite may not cause cancer and deformity, people
should still pay attention to their intake of nitrite since
no one has offered the perfect experiment and
research result to make sure that nitrite would not lead
to cancer, deformity, or maybe some other illness that
scientists have not found. Therefore, scientists should
continue to devote great efforts to the controversy or
carcinogenicity and teratogenicity of nitrite in the
future in order to understand the property of nitrite in
depth so that people can ingest nitrite properly, and
then achieve healthier life.
The influence of nitrite is only one part of food
safety. Food safety nowadays is a serious problem in
many countries. Thus, governments should introduce
new policies to control food products, and supervise
food hygiene; organizations and social medias should
popularize knowledge of food safety; individuals
should follow the instructions and pay more attention
to their everyday food. The more everyone devote to
food safety, the healthier people should be.
5 CONCLUSIONS
Nitrite has benefits of curing cardiovascular disease,
and defensing bacteria. What is more, it has
controversial harms of causing cancer and deformity,
and the doubtless harm of nitrite poisoning. To deal
with the benefits and harms of nitrite, people should
follow instructions to ingest nitrite properly, and
government need to take actions to reduce nitrite
pollution. Additionally, scientists should continue to
explore the carcinogenicity and teratogenicity of
nitrite, in order to make measures of controlling
nitrite more impeccable.
ACKNOWLEDGMENTS
I appreciate my teachers, Jane and Alice, and my
parents, who supported me helped me a lot. Without
their help and encouragement, I cannot complete this
paper.
REFERENCES
Bryan N S, Alexander D D, Coughlin J R, et al. (2012).
Ingested nitrate and nitrite and stomach cancer risk: An
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
204

updated review [J]. Food & Chemical Toxicology,
50(10):3646-3665.
Cosby K, Partovi K S, Crawford J H, et al. (2003). Nitrite
reduction to nitric oxide by deoxyhemoglobin
vasodilates the human circulation [J]. Nature Medicine,
9(12):1498-1505.
Dykhuizen R S, Frazer R, Duncan C, et al. (1996).
Antimicrobial effect of acidified nitrite on gut
pathogens: importance of dietary nitrate in host defense
[J]. Antimicrobial Agents and Chemotherapy,
40(6):1422-1425.
Feng A L. (1983). The transformation of nitrate and nitrite
in human’ s body [J]. Foreign Medical Sciences
Hygienics, (5).
Griesenbeck J S, M D Steck, Huber J C, et al. (2009).
Development of estimates of dietary nitrates, nitrites,
and nitrosamines for use with the short willet food
frequency questionnaire [J]. Nutrition Journal, 8(1):16.
Huang C S, Xu J H, Qin M Z, et al. (2009). Relationship
between nitrite and cancer [J]. Journal of Henan
University(Nature version), (01):35-41.
Johnson G I, Tsao P S, Mulloy D, et al. (1990).
Cardioprotective effects of acidified sodium nitrite in
myocardial ischemia with reperfusion [J]. Journal of
Pharmacology and Experimental Therapeutics,
252(1):35-41.
Machha A , Schechter A N. (2011). Dietary nitrite and
nitrate: a review of potential mechanisms of
cardiovascular benefits[J]. European Journal of
Nutrition, 50(5):293-303.
Naseem K M. (2005). The role of nitric oxide in
cardiovascular diseases [J]. Molecular Aspects of
Medicine,26(1-2):33-65.
Nitrate and nitrite in the diet: How to assess their benefit
and risk for human health [J]. Molecular Nutrition &
Food Research. (2015) 59(1):106-128.
Nitrite, nitrite alternatives, and the control of Clostridium
botulinum in cured meats. Crit. Rev. Food Sci. Nutr.
17:141-187.
Program N. (2008). NTP Technical report on the toxicology
and carcinogenesis studies of sodium dichromate
dihydrate in F344/N rats and B6C3F1 mice (drinking
water studies). p.243.
Ren J, Tao L, Du Z. (2007). The influence of time of water
dispenser using on the quality of barrels drinking water
[J]. Journal of environment and health, 24(008):606-
607.
Smith S S, Schwarz, et al. (2006). Gastric DNA damage
through tobacco chewing: In vitro mechanistic studies
of DNA nitrite attack [J]. CANCER LETT, 235(2)(-
):221-228.
Sobko T, Marcus C, Govoni M, et al. (2010). Dietary nitrate
in Japanese traditional foods lowers diastolic blood
pressure in healthy volunteers [J]. Nitric Oxide,
22(2):136-140.
Webb A, Bond R, Mclean P, et al. (2004). Reduction of
nitrite to nitric oxide during ischemia protects against
myocardial ischemia eperfusion damage [J].
Proceedings of the National Academy of ences of the
United States of America.
Ye C. (2007). The pollution of nitrate and nitrite in
vegetables [J]. Food Engineering.
Zhang Y Q, Shen J Y, Ying-Ru X U, et al. (2015). Harm of
nitrite to human body and progress on test methods [J].
Occupation and Health.
Zhang Z, Lu C, Sun C X. (2017). Test result analysis of the
content of nitrite in cooked meat product in Zhoukou
during 2014-2015 [J]. Engineering of public health in
China, 016(004):451-452.
Analysis of the Influence of Nitrite for Public Health
205
