
Microorganism Coculture-independent Synthesis of
Berkeleypenostatin A
Kun Wei
Dulwich College Beijing, Beijing, 101300, China
Keywords: Retrosynthesis, Berkeleypenostatin, Anti-Cancer, Microorganism Coculture-independent.
Abstract: In 2021, berkeleypenostatins A-G have been biosynthesized by the coculture fermentation of microbes -
Penicillium fuscum and P. camembertii/clavigerum - isolated from the Berkeley Pit Lake (Stierle, Stierle,
Decato, Alverson, Apedaile, 2021). Tested by the NCI Developmental Therapeutics Program,
berkeleypenostatin A effectively inhibited the migration of human pancreatic carcinoma cells (HPAF-II)
(Stierle, Stierle, Decato, Alverson, Apedaile, 2021). However, despire the potent anti-tumor activity
demonstrated by berkeleypenostatin A, its production from terrestrial extremophilic fungi presents challenges
such as low-cell growth and high shear sensitivity (Ludlow, Clark 1991). Considering the potential of
berkeleypenostatin A in pancreatic cancer treatment, this report proposes a laboratory synthesis of
berkeleypenostatin A as an alternative to fungal coculture. The report analyzes berkeleypenostatin A’s
common atoms, deduces its retrosynthesis disconnections, and plans an effective synthesis route. To
maximize the convergency of the synthesis, it begins with the reaction between a strong nucleophile and
electrophile, proceeds to the McMurry coupling and the Diels-Alder reaction, and ends with the addition of
glucose. Such a universal and simple synthesis introduces a series of rapid steps in producing
berkeleypenostatin A, a potential anti-cancer material, which offers an innovative insight to the future
treatment of pancreatic cancer.
1 INTRODUCTION
With a 5-year survival rate of 10%, pancreatic ductal
adenocarcinoma (PDAC) is leading the cancer-
related deaths worldwide (Osuna de la Peña, D.,
Trabulo, S.M.D., Collin, E. et al. 2021). Most patients
have advanced or metastatic disease at diagnosis
(Park, W., Chawla, A., & O’Reilly, E. M. 2021).
Existing treatments, including gemcitabine and nab-
paclitaxel, have limited efficacies due to various
complex factors affecting PDAC, such as
desmoplasia and hypervascularization (Osuna de la
Peña, D., Trabulo, S.M.D., Collin, E. et al. 2021).
Although chemotherapy with gemcitabine is the
standard therapy for advanced or metastatic disease,
its efficacy is highly limited by undesirable qualities
of rapid plasma degradation, toxicity, and drug
resistance (Tada 2011, Correia, Xavier, Duarte,
Ferreira, Moreira, Vasconcelos, Vale 2020).
Meanwhile, nab-paclitaxel demonstrates multiple
adverse side-effects, including alopecia, neutropenia
and nausea (Vishnu, Roy 2011). Therefore, there is
an urgent demand for developing effective
therapeutics for pancreatic cancer.
As a recently isolated compound,
berkeleypenostatin A displays the anti-tumor
qualities for a potentially effective therapeutic. The
structure and configuration of berkeleypenostatins A-
G have been deduced from spectral data and single-
crystal X-ray crystallography (Stierle, Stierle,
Decato, Alverson, Apedaile, 2021). After being
produced in coculture, berkeleypenostatins were
tested for anti-cancer activity. Among these
molecules, berkeleypenostatin A was identified as a
moderate inhibitor (50−100 μM) of MMP-3, an
enzyme that promotes metastasis in pancreatic tumor
cells (Suhaimi, Chan, Rosli 2020, Yang et al 2020).
Berkeleypenostatin A also induces reduced cell
migration of human pancreatic carcinoma cells
(HPAF-II) by 30% at a concentration of 1.25 μM over
a 24 h period (Stierle, Stierle, Decato, Alverson,
Apedaile, 2021). The results shed light on the
research for pancreatic cancer, which has been a
challenging issue with patients exhibiting a low
survival rate within five years after diagnosis and
Wei, K.
Microorganism Coculture-independent Synthesis of Berkeleypenostatin A.
DOI: 10.5220/0011296500003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 797-802
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
797

diagnosed in the advanced stages (Rawla, Sunkara,
Gaduputi 2019, American Cancer Society. 2021).
Although the current results indicate its potential in
cancer treatment, further biological assays are
essential to understanding more about the biological
activity of berkeleypenostatin A. Such inquiry
introduces the need to prepare berkeleypenostatin A
for analysis and evaluation.
However, the existing synthesis of
berkeleypenostatin A comes with various drawbacks
introduced by the complexity and time-consuming
nature of fungal coculture. Berkeleypenostatins are
examples of secondary metabolites grown in axenic
culture from microorganisms in the Berkeley Pit,
acidic metal-rich waste lake (Giddings, Newman
2015). To combat the inconveniences of producing
berkeleypenostatin A from microorganism coculture,
this report proposes a coculture-independent method
to synthesize berkeleypenostatin A with simple and
fast steps. It is expected to prepare berkeleypenostatin
A using the devised synthesis outlines below.
Figure 1. Structure of Berkeleypenostatin A
2 METHOD
As shown in Figure 1, the core of berkeleypenostatin
A has 11 stereocenters, which results in 2
11
potential
stereoisomers. Therefore, the final products of the
synthesis may include stereoisomers of this target
molecule. To maximize the simplification and
convergency of each retrosynthetic step, the strategy
is to disconnect bonds linking two or more common
atoms of the polycyclic core.
Figure 2. Retrosynthesis Strategy of Berkeleypenostatin A.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
798
After evaluating several retrosynthesis methods,
the final version, with the highest expected
efficiency, is presented in Figure 2. The
retrosynthesis is proposed to begin with a hydrolysis
reaction under acidic conditions, separating the R
group, β-D-glucose, from the rest of
berkeleypenostatin A 1. Berkeleypenostatin B 2 is
expected to undergo a Diels-Alder reaction to
disassemble the fused rings resulting in a bridged
bicyclic ring system composed of a pyrone and a 10-
carbon ring. Followed by a McMurry coupling, the
alkene group of molecule 3 will split into two chains
ending with an aldehyde group. When the 1,4
dioxygenated system of an ether group and an
aldehyde group cleave, molecules 5 and 6 will be
produced. These synthesized molecules will react as
the starting materials of berkeleypenostatin A.
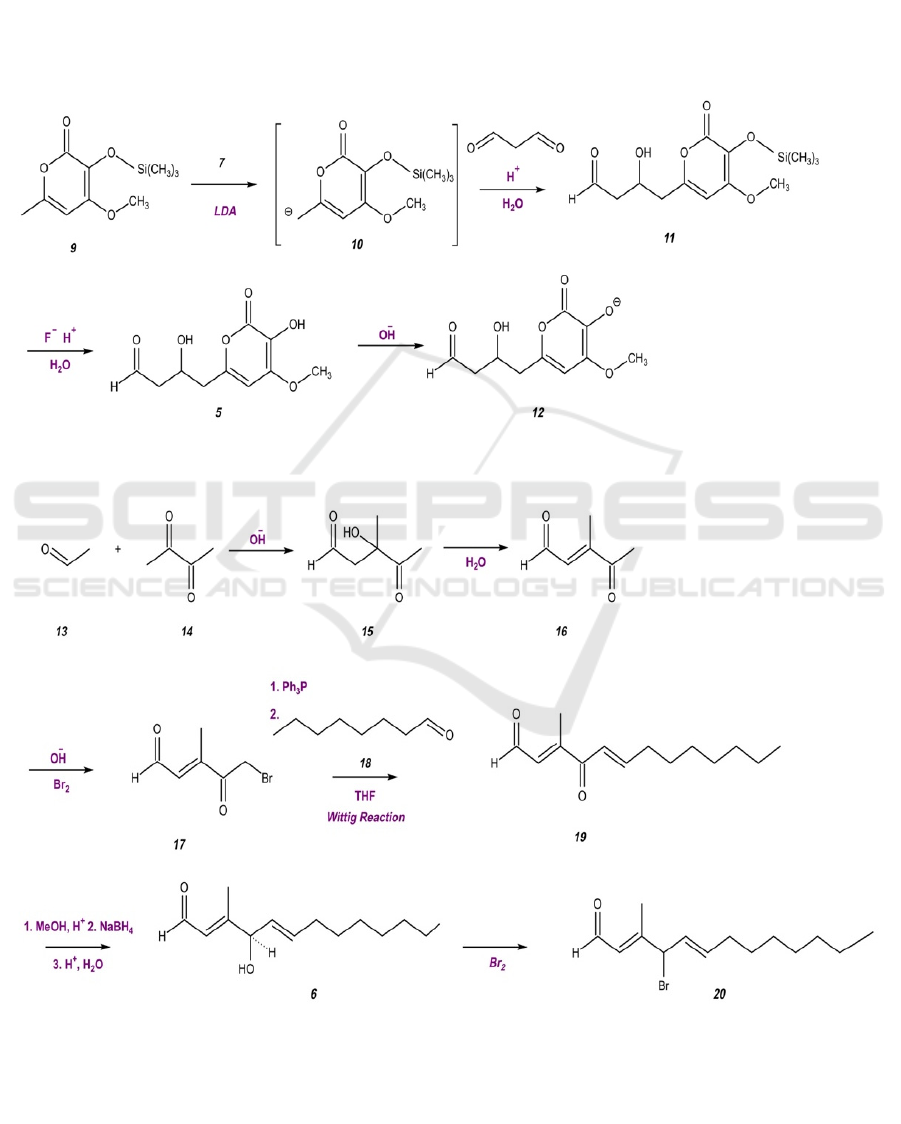
Figure 3. Preparation of Molecule 5 as an Improved Nucleophile.
Figure 4. Preparation of Molecule 6 as an Improved Electrophile.
Before the formal synthesis, molecules 5 and 6 are
prepared and tuned using the procedure presented in
Figures 3 and 4. The hydroxyl groups on molecule 5
are reactive, which may later interfere with essential
Microorganism Coculture-independent Synthesis of Berkeleypenostatin A
799

steps in the synthesis. Therefore, the hydroxyl groups
need to be masked with protective agents with
varying strengths. With lithium diisopropylamide
(LDA), molecule 9 forms a carbanion, which will
react with malonaldehyde under acidic conditions
with the existence of water to form molecule 11.
Under these conditions, the weak protective agent on
molecule 11, the silyl ether, will eventually be
converted to a hydroxyl group, forming molecule 5.
Under alkaline conditions, the hydroxyl group on
molecule 5 will be ionized to form an oxygen anion,
resulting in a stronger nucleophile.
Molecule 6 cannot be found as a commercial
reagent, so it is synthesized using simpler, more
accessible molecules. The starting materials,
acetaldehyde 13 and 2,3-butanedione 14, will join
together to form 2,5-hexanedione 15 under alkaline
conditions. 2,5-Hexanedione 15 will undergo a
functional group interconversion by eliminating
water, which transforms the hydroxyl group to an
alkene group. Next, a bromide ion will be added to 3-
Hexene-2,5-dione 16 using bromine liquid under
strong alkaline conditions. In the Wittig reaction, the
phosphorus of triphenylphosphine (Ph
P) attacks the
carbon next to the bromide group in molecule 17,
forming a ylide that reacts with heptaldehyde 18,
generating molecule 19. The ketone group on
molecule 19 is then converted to a hydroxyl group,
forming molecule 6. To improve the electrophilic
properties of molecule 6, the hydroxyl group is
converted to a bromide group. The enhanced
nucleophilic and electrophilic properties of molecules
20 and 12, respectively, prepare for their reaction.
Figure 5. Synthesis Strategy of Berkeleypenostatin A.
The steps to synthesizing berkeleypenostatin A
are shown in Figure 5. Molecules 12 and 20 will be
mixed under alkaline conditions, forming molecule 4.
With titanium chloride acting as a reducing agent, the
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
800

two aldehyde groups at the ends of molecule 4 will
connect to form an alkene group, undergoing the
McMurry reaction. The bicyclic bridged system of
pyrone 3 will fuse to form three fused rings under
heat, carrying out the Diels-Alder reaction. Finally,
by eliminating water via heating, β-D-glucose may be
added to berkeleypenostatin B 2 in a condensation
reaction, synthesizing berkeleypenostatin A.
3 RESULTS & DISCUSSION
By following the synthesis proposal,
berkeleypenostatin A is expected to be produced with
a few challenges. In the Wittig reaction, the aldehyde
group on the heptaldehyde may be affected given that
it is more reactive than the ketone group on molecule
17, producing an undesired outcome as the aldehyde
group converts into a hydroxyl group. If such
possibility is verified by experiment that it hugely
impacts the result, an alternative step should be
devised.
A notable limitation of the synthesis is the
production of undesired stereochemical outcomes.
For instance, when the malonaldehyde is added to
molecule 10, the product consists of one stereocenter,
forming two enantiomers of molecule 11 in a 1:1
ratio. Therefore, it is expected that
berkeleypenostatin A will be mixed with some
unexpected stereoisomers in the final products.
To boost the yield of berkeleypenostatin A, the
future direction of the synthesis is to develop an
enantioselective strategy of adding the
malonaldehyde to molecule 10, which may involve
the use of a catalyst.
4 CONCLUSION
In conclusion, this report suggests a synthesis
strategy of berkeleypenostatin A with significantly
maximized convergency. The logistics of the route
may be improved by experiments and testing the
optimal temperatures at different stages of the
synthesis, including the necessary heating during the
Diels-Alder reaction and the condensation reaction.
The yield of the synthesis may be enhanced by
stereoselective steps. Nevertheless, the report devised
a strategic laboratory method to synthesize
berkeleypenostatin A by reacting a nucleophile with
an electrophile in a minimal number of steps. Overall,
this microbe coculture-independent synthesis route of
berkeleypenostatin A has high prospects, offering a
new way of producing this anti-tumor reagent without
limitations of fungal coculture. Such a facile and
universal synthesis of berkeleypenostatin A would
make the material more readily available for
biological assays in evaluating its efficacy for
pancreatic cancer treatment.
REFERENCES
American Cancer Society. (2021). Survival Rates for
Pancreatic Cancer. American Cancer Society.
https://www.cancer.org/cancer/pancreatic-
cancer/detection-diagnosis-staging/survival-rates.html.
Correia, C., Xavier, C. P., Duarte, D., Ferreira, A., Moreira,
S., Vasconcelos, M. H., & Vale, N. (2020).
Development of potent CPP6–gemcitabine conjugates
against human prostate cancer cell line (PC-3). RSC
Medicinal Chemistry, 11(2), 268–273.
https://doi.org/10.1039/c9md00489k
Giddings LA., Newman D.J. (2015) Bioactive Compounds
from Terrestrial Extremophiles. In: Bioactive
Compounds from Terrestrial Extremophiles.
SpringerBriefs in Microbiology. Springer, Cham.
https://doi.org/10.1007/978-3-319-13260-0_1
Ludlow, J. M., & Clark, D. S. (1991). Engineering
Considerations for the Application of Extremophiles in
Biotechnology. Critical Reviews in Biotechnology,
10(4), 321–345.
https://doi.org/10.3109/07388559109038214
Osuna de la Peña, D., Trabulo, S.M.D., Collin, E. et al.
Bioengineered 3D models of human pancreatic cancer
recapitulate in vivo tumour biology. Nat Commun 12,
5623 (2021). https://doi.org/10.1038/s41467-021-
25921-9
Park, W., Chawla, A., & O’Reilly, E. M. (2021). Pancreatic
cancer. JAMA, 326(9), 851.
https://doi.org/10.1001/jama.2021.13027
Rawla, P., Sunkara, T., & Gaduputi, V. (2019).
Epidemiology of Pancreatic Cancer: Global Trends,
Etiology and Risk Factors. World Journal of Oncology,
10(1), 10–27. https://doi.org/10.14740/wjon1166
Stierle, A. A., Stierle, D. B., Decato, D., Alverson, J., &
Apedaile, L. (2021). Cryptic Biosynthesis of the
Berkeleypenostatins from Coculture of Extremophilic
Penicillium sp. Journal of Natural Products, 84(5),
1656–1665.
https://doi.org/10.1021/acs.jnatprod.1c00248
Suhaimi, S. A., Chan, S. C., & Rosli, R. (2020). Matrix
Metallopeptidase 3 Polymorphisms: Emerging genetic
Markers in Human Breast Cancer Metastasis. Journal
of Breast Cancer, 23(1), 1–9.
https://doi.org/10.4048/jbc.2020.23.e17
Tada, M. (2011). Recent progress and limitations of
chemotherapy for pancreatic and biliary tract cancers.
World Journal of Clinical Oncology, 2(3), 158.
https://doi.org/10.5306/wjco.v2.i3.158
Vishnu, P., & Roy, V. (2011). Safety and efficacy of NAB-
paclitaxel in the treatment of patients with breast
Microorganism Coculture-independent Synthesis of Berkeleypenostatin A
801

cancer. Breast Cancer: Basic and Clinical Research, 5,
53–65. https://doi.org/10.4137/bcbcr.s5857
Yang, J., Antin, P., Berx, G., Blanpain, C., Brabletz, T.,
Bronner, M., Campbell, K., Cano, A., Casanova, J.,
Christofori, G., Dedhar, S., Derynck, R., Ford, H. L.,
Fuxe, J., García de Herreros, A., Goodall, G. J.,
Hadjantonakis, A.-K., Huang, R. J., Kalcheim, C., …
Sheng, G. (2020). Guidelines and Definitions for
Research on Epithelial–Mesenchymal Transition.
Nature Reviews Molecular Cell Biology, 21(6), 341–
352. https://doi.org/10.1038/s41580-020-0237-9
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
802
