
Lipoproteins and Atherosclerotic Cardiovascular Diseases
Jingying Wang
a
University of California, Los Angeles, U.S.A.
Keywords: Lipoprotein, Atherosclerosis, Animal Models, Statistic Models.
Abstract: Metabolisms of triglycerides and cholesterol from both the intestine and the liver are important in humans.
Generally, there are three lipoprotein pathways involving chylomicrons, chylomicron remnants, VLDL, LDL,
and HDL. Started in arterial endothelium, accumulation of LDL and proteoglycans will form oxidized LDL
and lead to arterial macrophages. Then, macrophage foam cells will enhance plaque progression. If the plaque
is unstable, it is very likely to develop plaque rupture and form thrombus. To better understand the mechanism
and find medications, it is important to choose adequate animal models based on lipid metabolism and
characteristics of atherosclerosis. The combination of statistical models in recent studies helps to examine the
effects of two independent factors on one dependent factor.
1 INTRODUCTION
Nowadays, people consume more and more food
containing high fat and cholesterol in developed
countries and the likelihood of getting atherosclerosis
increases. To attenuate or avoid the symptoms of
atherosclerotic cardiovascular diseases, it is
necessary to understand the principles behind these
diseases. In addition, the combination of statistical
models to biological research may differentiate the
influence of two independent factors or two
dependent factors, which provide futural experiments
with possibilities to include more factors in one
research.
2 LIPOPROTEINS, RECEPTORS,
ENZYMES, AND
LIPOPROTEIN PATHWAYS
Lipoproteins are complexes with hydrophobic core,
which is formed by triglyceride and cholesteryl
esters, and hydrophilic phospholipids, free
cholesterol, and apolipoproteins. There are seven
classes of lipoproteins: chylomicrons, chylomicron
remnants, very low density lipoproteins (VLDL),
intermediate density lipoproteins (IDL), low density
a
https://orcid.org/0000-0002-2023-7801
lipoproteins (LDL), high density lipoproteins (HDL),
and lipoprotein (a) (Lp (a)).
Chylomicrons are triglyceride-rich particles
produced by the intestine. The major structural
protein of chylomicrons is apolipoprotein B-48 (Apo
B-48) that cannot be recognized by LDL receptors.
Chylomicron remnants are smaller particles after
removing triglycerides from chylomicrons by
peripheral tissues. These smaller particles are high in
cholesterol and more pro-atherogenic. VLDL are
triglyceride-rich particles produces by the liver, and
they are smaller than chylomicrons. Compared with
chylomicrons, the major structural protein is Apo B-
100 which is a ligand for LDL receptors. IDL, or
VLDL remnants, is a smaller particle after removing
triglycerides by muscle and adipose tissue. Similar to
chylomicron remnants, IDL is enriched in cholesterol
and pro-atherogenic. LDL is derived from VLDL and
IDL, and it is more enriched in cholesterol. Smaller
LDL is more pro-atherogenic than larger LDL
because it has a decreased affinity with LDL
receptors, which leads to a longer retention time, and
they bind more tightly to proteoglycans and are more
likely to be oxidated, which increases the
consumption by macrophages. (Feingold 2021) HDL
is anti-atherogenic because it acts in reverse
cholesterol transport. The major structural protein of
HDL is Apo A-I. Apo A-I helps interaction between
HDL and ATP-binding cassette transport A1
404
Wang, J.
Lipoproteins and Atherosclerotic Cardiovascular Diseases.
DOI: 10.5220/0011371300003438
In Proceedings of the 1st International Conference on Health Big Data and Intelligent Healthcare (ICHIH 2022), pages 404-409
ISBN: 978-989-758-596-8
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reser ved

(ABCA1) and ABCG1 that transport cholesterol from
cells to HDL in reverse cholesterol transport.
(Feingold, 2021) Lp (a) is an LDL particle with
apolipoprotein (a) and it is pro-atherogenic. Another
important apolipoprotein is Apo C that is found in
chylomicrons, VLDL, and HDL. Apo C-II is a co-
factor of lipoprotein lipase (LPL) and hydrolyzes
triglycerides, which means that it is anti-atherogenic.
However, Apo C-III is an inhibitor of LPL and
inhibits triglycerides interact with their receptors.
In addition to ABCA1 and ABCG1, several
receptors and transporters are important in lipid
metabolism. LDL receptors determine the LDL level
in the plasma: the high level of LDL receptors is
corresponding to a low LDL level, while the low level
of LDL receptors is corresponding to a high LDL
level. (Feingold 2021) With the delivery of
cholesterol to cells, the level of HMG CoA reductase
decreases, and the level of acyl-CoA cholesterol acyl
transferase (ACAT) increases. As a result, the level of
LDL receptors, which are controlled by HMG CoA
reductase, decreases. When the cellular cholesterol
level is low, the transcription factor SREBP will be
activated, and LDL receptors will be stimulated to
express. In contrast, when the cellular cholesterol
level is high, the SREBP will be inactive, and the
expression of LDL receptors is low. (Feingold 2021)
Similar to ABCA1, AND ABCG1, class B scavenger
receptor B1 (SR-B1) facilitates selective uptake of
cholesterol esters from HDL particles.
There are several enzymes involved in lipoprotein
metabolism. Lipoprotein lipase (LPL) hydrolyzes
triglycerides in chylomicrons and VLDL and forms
chylomicron remnants and IDL. Apo C-II and Apo A-
V are cofactors of LPL, while Apo C-III and Apo A-
II are inhibitors. Lecithin cholesterol acyltransferase
(LCAT) catalyzes the synthesis of cholesterol esters
and transfers free cholesterol from the surface of the
HDL particle to the core and forms cholesterol esters.
(Feingold 2021) This process reduces the
concentration of cholesterol on the HDL particles and
allows the consumption of more cholesterol from
cells. Cholesteryl ester transfer protein (CETP) helps
transfer cholesterol esters from HDL to VLDL,
chylomicrons, and LDL, and triglycerides from
VLDL and chylomicrons back to HDL. (Feingold
2021)
There are three major lipoprotein pathways:
exogenous lipoprotein pathway, endogenous
lipoprotein pathway, and reverse cholesterol
transport. Exogenous lipoprotein pathway starts in
the intestine: dietary lipids are incorporated into
chylomicrons. Triglycerides in chylomicrons are
hydrolyzed into muscle and adipose tissue into free
fatty acids by LPL, and chylomicrons form
chylomicron remnants. (Feingold 2021) Endogenous
lipoprotein pathway starts in the liver with VLDL.
Triglycerides in VLDL are hydrolyzed into free fatty
acids, and VLDL forms IDL. IDL can be further
metabolized into LDL and can be consumed in tissues
via LDL receptors. (Feingold 2021) Reverse
cholesterol transport begins with nascent HDL in the
liver and intestine. Cells donate cholesterol and
phospholipids via ABCA1 to nascent HDL and form
mature HDL. Additional cholesterol can be
transferred to mature HDL via ABCG1, SR-B1, or
passive diffusion. (Feingold 2021) Facilitated by
CETP, HDL transports cholesterol back to the liver
via SR-B1 or indirectly to VLDL or LDL.
3 ATHEROSCLEROTIC
CARDIOVASCULAR DISEASE
Accumulation of LDL, which is one of the major
culprits of atherosclerotic cardiovascular disease, is
due to interaction between positive-charged amino
acyl residues in Apo B-100 with negative-charged
sulfate and carboxylic acid groups of proteoglycans
in the artery wall. Both changes in the core or on the
surface of LDL may enhance atherosclerosis. For
example, enrichment of cholesterol in LDL or Apo E,
Apo C-III, and serum amyloid A can increase the
binding affinity of LDL and arterial wall
proteoglycans. (Borén 2020) In humans, there is an
inclination to develop atherosclerosis at branches and
bifurcations with laminar blood flow and low or
fluctuating shear stress.
There are four subfractions of LDL: large LDL-I,
LDL-II with intermediate size and density, small
LDL-III, and very small LDL-IV. People with
medium plasma triglyceride levels will release
VLDL1 and VLDL2 that is further metabolized into
LDL-II; people with low plasma triglyceride levels
mainly release smaller VLDL and form
predominantly LDL-I, along with some LDL-II;
people with high plasma triglyceride level release
dense LDL-IV due to high level of VLDL, and they
generally lack lipolysis due to inhibition of
overproduced Apo C-III on LPL. (Borén 2020) Small
dense LDL is more pro-atherogenic than larger LDL
because it enters the artery faster and has a longer
retention time due to impaired binding affinity to
LDL receptors. In addition, small dense LDL is
enriched in Apo C-III and glycated Apo B, and
unsaturated cholesteryl esters are more susceptible to
hydroperoxide. (Borén 2020)
Lipoproteins and Atherosclerotic Cardiovascular Diseases
405

With a longer retention time, LDL particles are
more likely to form oxidized LDL and trigger the
entrance of monocytes into the artery. Monocytes
differentiate in the artery and become macrophages
and intensify oxidized LDL that can be consumed by
scavenger receptors like clusters of differentiation-36
(CD36) and form foam cells. Modified LDL triggers
a series of innate and adaptive immune responses and
leads to inflammation. Defective efferocytosis, which
results in non-resolving inflammation, is due to
signals like CD47 in the artery and will lead to the
accumulation of cell debris. (Borén 2020) Apoptotic
cell will stimulate secondary necrosis that results in
unstable plaque, plaque rupture, and later thrombus
formation. Both plaque rupture and plaque erosion
may lead to thrombus formation. With lipid cores or
thin fibrous cap tissue between the lipid core and
blood that reaches the luminal surface, the blood can
enter and core material may leak out. (Borén 2020)
This process forms plaque rupture that always
accompanying by protruding cholesterol crystals. In
contrast, lesions without lipid cores or thick fibrous
cap will not lead to plaque rupture but instead plaque
erosion, where the plaque is intact but endothelial
cells are deficient.
1
Recent researches show that a
spotty pattern of calcium deposits is prone to be more
dangerous. An elevated LDL-cholesterol level is one
of the risk factors of calcification. (Borén 2020) In
contrast, HDL-mediated efflux of cholesterol inhibits
calcification. (Borén 2020) While the formation of
atherosclerosis is attributed to the accumulation of
oxidated LDL, the relation between lowering
aggressive LDL and lesion area remains unclear.
Similarly, while HDL features anti-inflammatory and
anti-oxidative functions, its role in attenuating lesion
areas is indistinct but probable.
4 CHOICE OF ANIMAL MODELS
Since it is impossible to track the lengthy
development of atherosclerosis in arteries of humans,
it is necessary to observe that in animal models which
are representative of humans. The murine model is
ideal because of its small size and its relative
homogeneity to humans. For example, both the
mechanism of triglyceride-rich lipoprotein inducing
atherosclerosis and Apo A-I lowering atherosclerosis
can be applied to humans. (Daugherty 2017)
However, while a murine transports cholesterol
primarily in HDL, humans utilize LDL. This
difference in lipoprotein profile protects a murine
from atherosclerosis because there is no binding site
of Lp-PLA2 to LDL. Besides, there is a lower
probability to form oxidated LDL and trigger
atherosclerosis, but a higher probability to form
stable plaques. Furthermore, the much higher level of
LDL receptors in the liver in a murine than in humans
leads to lower LDL levels in a murine and a lower
probability to develop atherosclerosis. There is a
lower level of plasma Apo B on LDL in a murine than
in humans. The chylomicron is from the intestine, and
the VLDL is from the liver in humans. However, Apo
B-48 exists in the VLDL, and Apo B-100 exists in
chylomicrons in a murine. Fed with a high-fat high
cholesterol diet, humans are prone to develop
increased plasma cholesterol and triglycerides, while
a murine may develop increased plasma cholesterol
but lowered triglycerides. In addition, the murine
accumulates lesions primarily in the aortic root, arch,
and other side branches instead of coronary arteries
in humans. Recent studies mainly utilize either Apo
E knock-out mice or LDL receptor knock-out mice.
Although Apo E knock-out mice carry more VLDL,
LDL receptor knock-out mice have higher LDL
particles that are more atherogenic. (Getz, & Reardon
2016) However, Apo E has some athero-protective
functions other than lower plasma lipids like anti-
inflammation and anti-oxidation to lower
atherosclerosis, which generalizes how Apo B-
containing lipoproteins influence atherosclerosis
more difficult. (Getz, & Reardon 2016)
While it is
convenient to study characteristics of atherosclerosis
in a murine, there are limitations when applying the
same mechanism to humans.
Pigs are more relevant to humans than murine.
Like humans, pigs transport cholesterol primarily in
LDL, and pigs also have a binding site of Lp-PLA2
to LDL. Moreover, similar to humans, the LDL
receptors level is low in pigs, and a high-fat high
cholesterol diet can stimulate the increase in both
plasma cholesterol and triglycerides. All these
properties determine the susceptibility of
atherosclerosis and unstable characteristics of
plaques. Pigs are inclined to develop lesions at
branches with low shear stress and laminar blood
flow including coronary, and it is more convenient to
observe changes in arteries because of the huge size
of pigs. (Daugherty 2017) However, since there is no
CETP in pigs, the mechanism of atherosclerosis in
pigs is different from that of humans. In addition, the
huge size of pigs will increase the cost of feeding and
increase the difficulty of experiments.
Recently, scientists find great similarities between
hamsters and humans. Hamsters utilize LDL to
transport cholesterol, and they have CETP, which
means that hamsters share a similar mechanism of
atherosclerosis development. Besides, most Lp-
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
406

PLA2 in hamsters binds to LDL instead of HDL, and
this increases the susceptibility of atherosclerosis and
forms unstable plaques. All these similarities with
humans, along with the small size of hamsters, ensure
that hamsters are one of the most appropriate animal
models when there is a need to how atherosclerosis
may develop in humans. However, information about
the place of atherosclerosis is still limited, and more
experiments about atherosclerosis in hamsters are
needed.
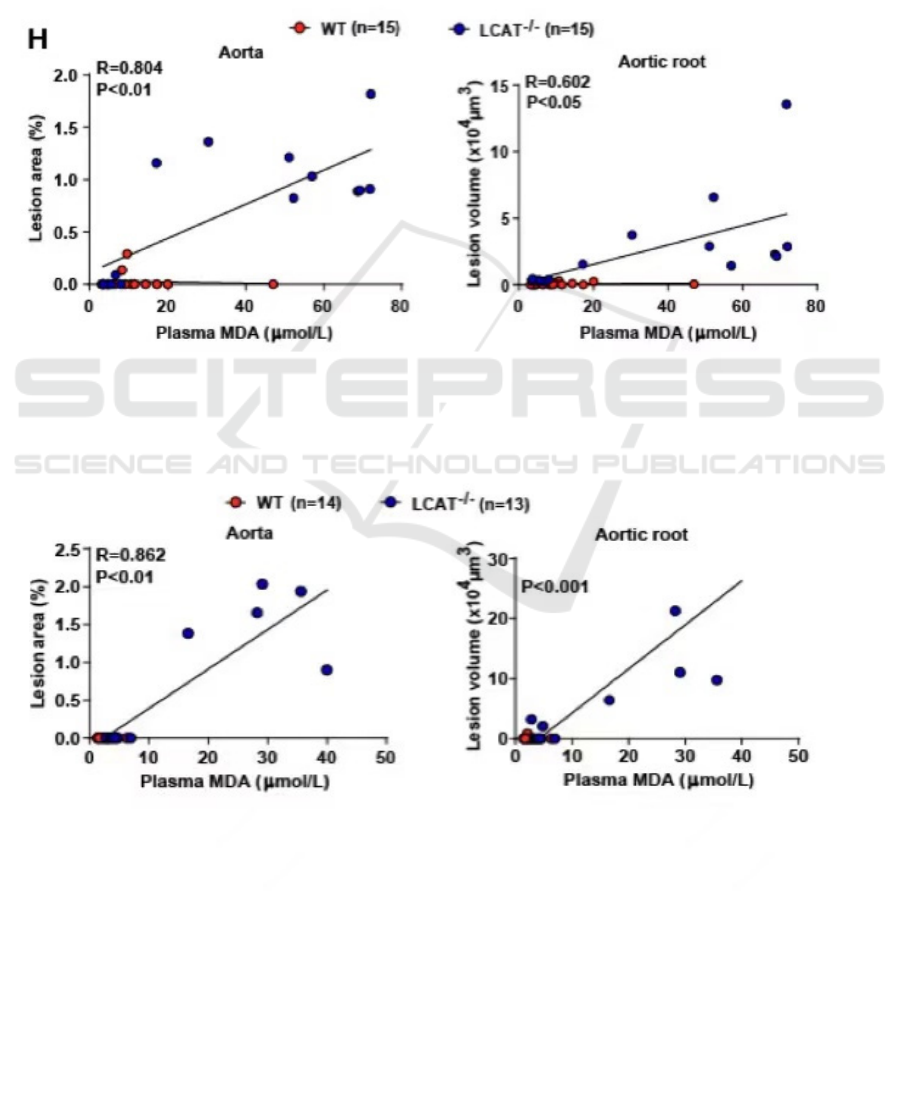
5 LCAT DEFICIENT HAMSTERS
AND STATISTIC MODELS
In recent scientific studies, it is common to combine
statistical models to analyze research results. In
research conducted by Guo, et al., it is noted that aged
male and female hamsters that are deficient in LCAT
develop atherosclerosis with higher plasma oxidative
lipids but not total cholesterols. (Guo, Liu, Xu, Ma,
Huang, Gao, Wang, Liu, & Xian 2020)
In the study,
Guo, et al. employ a two-way ANOVA test and linear
regression.
In linear regression, there are three important
values: total variability, remaining residue, and the
explained viability due to regression. Total
variability, or 𝑆𝑆
, can be written as Y − Ȳ, which
stands for the difference between the exact value Y
and the mean Ȳ. Remaining residue, or 𝑆𝑆
, can be
written as Y – Ŷ, and it stands for the difference
between the exact value Y and the value on the model
Ŷ. The explained viability due to regression can be
written as Ŷ − Ȳ, and it stands for the difference
between the value on the model Ŷ and the mean Ȳ.
Based on these three values, the coefficient of
determination R square in linear regression is defined
as:
𝑅
=1−
=1−
∑
(
)
∑
(
)
(1)
When 𝑅
is closer to 1, the linear model is more
accurate. In the study of Guo, et al., the linear model
fits well to samples. In the aorta of male
hamsters 𝑅 = 0.804 , and in female hamsters 𝑅 =
0.862 (Figure 1, 2). In the aortic root of male
hamsters, 𝑅 = 0.602 . Based on these data, it is
reliable to conclude that as the plasma
malondialdehyde (MDA) level increases, the lesion
volume, or the severity of atherosclerosis in the aorta
of LCAT deficient hamsters increases.
In the ANOVA test, a statistic F test uses F value
to compare two variances. First, the formula of
regression sum of squares (SSR) is:
𝑆𝑆𝑅 = 𝑛
∑
(𝑥
−𝑥
)
(2)
Where n is the sample size of group j; 𝑥
is the
mean of group j; and 𝑥
is the mean of all
samples.
Then, the formula of the error sum of squares
(SSE) is:
𝑆𝑆𝐸 =
∑∑
(𝑥
−𝑥
)
(3)
Where 𝑥
is the i
th
term in group j; and 𝑥
is the
mean of group j.
The total sum of squares (SST) is defined as:
𝑆𝑆𝑇 = 𝑆𝑆𝑅 + 𝑆𝑆𝐸. (4)
Finally, based on formulas (2), (3), and (4), the F
value in F test is:
𝐹=
=
=
(5)
Where k is the number of groups; and n is the total
sample size.
Based on the calculated F value and the table of
critical values of the F distribution, it is convenient to
find out the P-value that stands for the probability of
coincident results. If the calculated F value is larger
than the critical F value, then it is statistically
significant to reject the null hypothesis that the
variance between the means of two samples has no
significant difference. In the study of Guo et al., P-
values for aorta in male hamsters and aortic root in
female hamsters are less than 0.01, while the P-value
for aortic root in male hamsters is less than 0.05
(Figure 1, 2). In addition, a two-way ANOVA test is
applied to consider the influence of both gender and
age on lesion area in the study of Guo et al. Different
from the one-way ANOVA test, a two-way ANOVA
test considers two conditions: the influence of gender
only on the lesion area and the influence of age only
on the lesion area. Since all P-values for lesion areas
in the aorta and aortic root are less than 0.01 in male
hamsters and 0.001 in female hamsters, it is
statistically significant to conclude that LCAT
deficient hamsters tend to develop higher plasma
MDA levels and larger lesion areas than wild-type
hamsters (Figure 3, 4).
6 CONCLUSIONS
Recent studies develop some generalized ideas about
the characteristics of lipoproteins and their pathways
in transporting cholesterol. Defects in lipoproteins,
lipoprotein receptors, transporters, or enzymes will
Lipoproteins and Atherosclerotic Cardiovascular Diseases
407
lead to retention of LDL in the artery and the
formation of atherosclerosis. However, there are still
confusions about the functions of some
apolipoproteins like Apo C-III and Apo E in the
formations of atherosclerosis. Furthermore, since
current animal models of mice are not appliable to
humans in some cases, futural research about how
these defects express on other animal models like
hamsters may be insightful. When conducting
research, the employment of statistical models helps
to differentiate the influence of various independent
factors that will reduce potential errors when there are
several sets of variables.
7 SUPPLEMENT
Figure 1: The correlation of plasma MDA level and lesion area in aorta and aortic root in wild-type and LCAT deficient male
hamsters (adapted from Guo, et al. 2020).
Figure 2: The correlation of plasma MDA level and lesion area in aorta and aortic root in wild-type and LCAT deficient female
hamsters (adapted from Guo, et al. 2020).
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
408

Figure 3: Quantification of atherosclerosis lesion area in aortic roots in male hamsters; Quantification of atherosclerosis
plaque area in the aorta in male hamsters; Plasma MDA levels in male hamsters. Scale bar: 1mm. P<0.01 by Two Way
ANOVA/Bonferronis post-test (adapted from Guo, et al. 2020).
Figure 4: Quantification of atherosclerosis lesion area in aortic roots in female hamsters; Quantification of atherosclerosis
plaque area in the aorta in female hamsters; Plasma MDA levels in female hamsters. Scale bar: 1mm. P<0.001 by Two Way
ANOVA/Bonferronis post-test (adapted from Guo, et al. 2020).
REFERENCES
Borén, J., Chapman, M. J., Krauss, R. M., Packard, C. J.,
Bentzon, J. F., Binder, C. J., Daemen, M. J., Demer, L.
L., Hegele, R. A., Nicholls, S. J., Nordestgaard, B. G.,
Watts, G. F., Bruckert, E., Fazio, S., Ference, B. A.,
Graham, I., Horton, J. D., Landmesser, U., Laufs, U.,
Masana, L., … Ginsberg, H. N. (2020). Low-density
lipoproteins cause atherosclerotic cardiovascular
disease: pathophysiological, genetic, and therapeutic
insights: a consensus statement from the European
Atherosclerosis Society Consensus Panel. European
heart journal, 41(24), 2313–2330.
https://doi.org/10.1093/eurheartj/ehz962
Daugherty, A., Tall, A. R., Daemen, M., Falk, E., Fisher, E.
A., García-Cardeña, G., Lusis, A. J., Owens, A. P., 3rd,
Rosenfeld, M. E., Virmani, R., & American Heart
Association Council on Arteriosclerosis, Thrombosis
and Vascular Biology; and Council on Basic
Cardiovascular Sciences (2017). Recommendation on
Design, Execution, and Reporting of Animal
Atherosclerosis Studies: A Scientific Statement From
the American Heart Association. Circulation research,
121(6), e53–e79.
https://doi.org/10.1161/RES.0000000000000169
Feingold, K. R. (2021). Introduction to Lipids and
Lipoproteins. In K. R. Feingold (Eds.) et. al., Endotext.
MDText.com, Inc.
Getz, G. S., & Reardon, C. A. (2016). Do the Apoe-/- and
Ldlr-/- Mice Yield the Same Insight on Atherogenesis?.
Arteriosclerosis, thrombosis, and vascular biology,
36(9), 1734–1741.
https://doi.org/10.1161/ATVBAHA.116.306874
Guo, M., Liu, Z., Xu, Y., Ma, P., Huang, W., Gao, M., Wang,
Y., Liu, G., & Xian, X. (2020). Spontaneous
Atherosclerosis in Aged LCAT-Deficient Hamsters
With Enhanced Oxidative Stress-Brief Report.
Arteriosclerosis, thrombosis, and vascular biology,
40(12), 2829–2836.
https://doi.org/10.1161/ATVBAHA.120.315265
Lipoproteins and Atherosclerotic Cardiovascular Diseases
409
