
Determination of 4-Sulfonamidophenylhydrazine and Sulfonamide in
Diarylpyrazole Derivatives by LC-MS
Meng Yu
a
, Qing Sun
b
, Ling Sun
c
, Renyong Zhao
d
and Jinhu Wang
e
Shan Dong Academy of Pharmaceutical Sciences, Shandong Provincial Key Laboratory of Chemical Drug, Jinan, China
Keywords: LC-MS, Sulfonamide, 4-Sulfonamidophenylhydrazine, Diarylpyrazole Derivatives.
Abstract: A sensitive and selective LC-MS method was developed for the determination of
4-sulfonamidophenylhydrazine and sulfonamide in diarylpyrazole derivatives. The analysis of two
impurities was done on Inertsil Ph-3 phenyl column (100 mm×4.6 mm, 3 μm). The mobile phase was
gradient elution with 0.01 mol/L ammonium acetate buffer solution (pH 4.0) and methanol at a flow rate of
0.5 mL/min. Mass spectrometry adopts electrospray ion source, monitoring in positive ion mode. The limits
of quantification of 4-sulfonamidophenylhydrazine and sulfonamide were 0.4915 ng/mL and 0.5079 ng/mL,
respectively. They had a good linear relationship within their respective concentration ranges, and the
average recoveries were 106.96% and 106.71%, respectively. The method can be used for the determination
of 4-sulfonamidophenylhydrazine and sulfonamide in diarylpyrazole derivatives.
1 INTRODUCTION
1
As an important intermediate in the drug market,
pyrazole compounds are widely used in the
synthesis and development of drug targets, and are
the mainstream of current drug development (Zhong
2015, Selvam 2005, Bekhit 2004). Pyrazole
compounds have many pharmacological activities,
such as antibacterial, anti-inflammatory, anti-tumor,
etc (Zhang 2014, Tang 2008, Liu 2007). Some
pyrazole compounds have been developed into
marketed drugs or undergoing clinical research, such
as anti-inflammatory drug celecoxib, antibacterial
drug sulfafenpyrazole, antihypertensive drug
riociguat, anticancer drug anthrapyrazol, etc (Pathak
2012, Penning 1997, Ghofrani 2009).
Structure-activity relationship studies have shown
that the 1, 3, and 5 position substitutions of the
nucleus of pyrazole compounds play a key role in
the selectivity of NIAIDs, while the 4-position
substitution makes the selectivity to COX-2
decrease (Wang 2014, Stauffer 2000, Katoch 2003).
Synthesizers used the selective COX-2 inhibitor
a
https://orcid.org/0000-0002-7511-4294
b
https://orcid.org/0000-0002-5385-8276
c
https://orcid.org/0000-0002-9693-7003
d
https://orcid.org/0000-0003-2647-2826
e
https://orcid.org/0000-0003-2068-9928
celecoxib as a model compound to design and
synthesize multiple diaryl-substituted pyrazole
derivatives. The synthetic intermediate
4-sulfonamidophenylhydrazine contains a
genotoxicity warning structure, and its starting
material, sulfonamides, which is relatively toxic and
difficult to metabolize (Gong 2019, Baran 2011, Yu
2015), will generate azo compounds with
genotoxicity warning structure in subsequent
reactions. Therefore, a liquid mass spectrometry
method was established to determine the content of
4-sulfonamidophenylhydrazine and sulfonamide in
diarylpyrazole derivatives (Reddy 2015, Szekely
2015, Rajput 2017). The method was validated as
per ICH guidelines in terms of limit of detection
(LOD), limit of quantification (LOQ), linearity,
precision, accuracy, specificity, and solution
stability. See Table 1 for specific information.
992
Yu, M., Sun, Q., Sun, L., Zhao, R. and Wang, J.
Determination of 4-Sulfonamidophenylhydrazine and Sulfonamide in Diarylpyrazole Derivatives by LC-MS.
DOI: 10.5220/0011375300003443
In Proceedings of the 4th International Conference on Biomedical Engineer ing and Bioinformatics (ICBEB 2022), pages 992-998
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reser ved
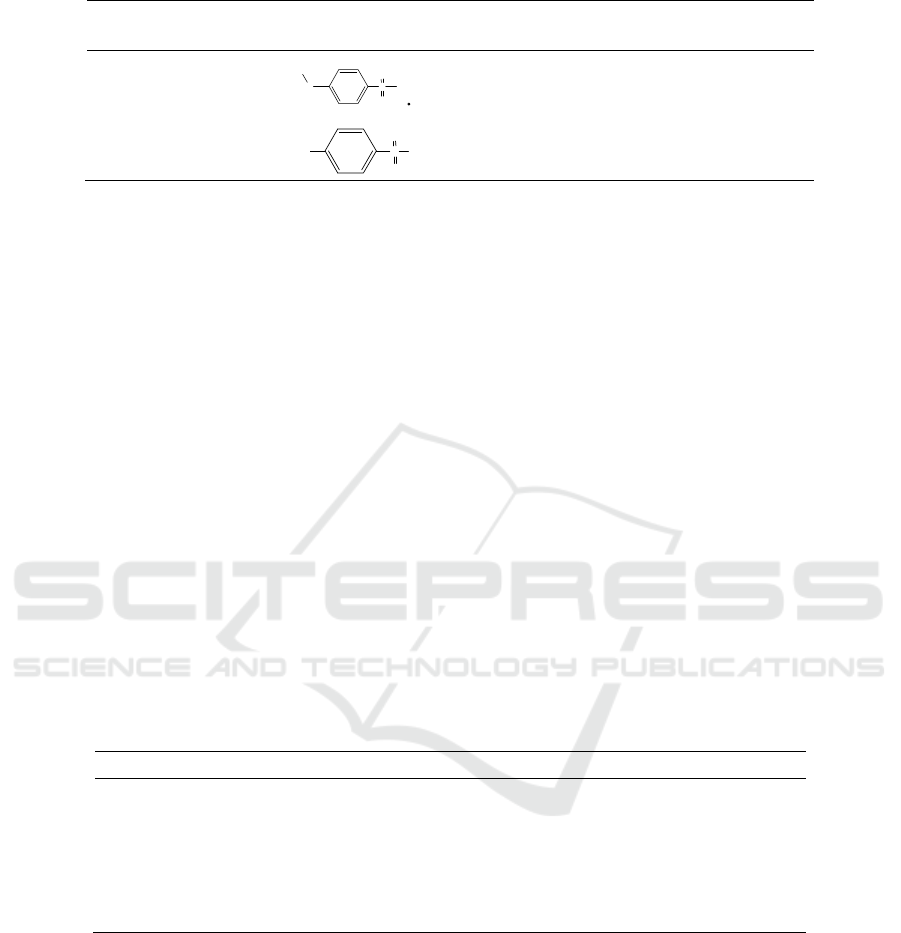
Table 1: Impurity information sheet.
Compound Chemical Structure
Molecular Formula/
Exact Molecule Weight
4-sulfonamidophe
nylhydrazine
C
6
H
9
N
3
O
2
S·HCl / 223.68
Sulfonamide
C
6
H
8
O
2
N
2
S/ 172.20
2 EXPERIMENTAL
2.1 Chemical and Reagents
HPLC grade acetonitrile was obtained from Concord
Technology, Tianjin, China. Analytical grade
ammonium acetate and HPLC grade acetic acid
were purchased from Sinopharm Chemical Reagent
Co., Ltd, China. Purified water was collected
through Milli-Q Plus water purification system.
Diarylpyrazole derivatives API was homemade.
Reference substances of
4-sulfonamidophenylhydrazine (99.5%) and
sulfonamide (99.2%) were supplied by Toronto
Research Chemicals, Canada.
2.2 Instrumentation
This research was performed on THERMO make
Ultimate 3000 UPLC-MS system. It has dual ternary
gradient pump, column oven with range of 5°C to
80°C with autosampler, diode array detector (UV)
and Q-Exactive Focus Orbitrap detector.
2.3 Chromatographic Conditions
Development and validation of the method were
carried on the LC-MS system. The analytical
column was Inertsil Ph-3 phenyl column (100
mm×4.6 mm, 3 μm) in gradient mode using 0.01
mol/L ammonium acetate buffer solution (pH 4.0)
and methanol (Table 2). The flow rate was 0.5
mL/min. The column temperature was maintained at
25℃, and the injection volume was 5 μL. The
effluent did not enter the mass spectrometer after 5
minutes controlled by the switching valve.
Table 2: Gradient programme.
Time (min) Ammonium acetate buffer (%) Methanol (%)
0.0 60.0 40.0
4.0 60.0 40.0
5.0 20.0 80.0
15.0 20.0 80.0
15.1 60.0 40.0
20.0 60.0 40.0
2.4 Mass Spectrometer
The MS system used was an Q-Exactive Orbitrap
mass spectrometer with electrospray ionization
probe operated in positive polarity. Selected Ion
Monitoring mode was chosen for the quantification
of 4-sulfonamidophenylhydrazine and sulfonamide.
4-sulfonamidophenylhydrazine was monitored with
its molecular ion [M+Na]+m/z 210.03077, and
sulfonamide was monitored with its molecular ion
[M+Na]+m/z 195.01987 in this method (Figure 1).
Typical operating conditions were as follows:
capillary temperature 320℃, aux gas heater
temperature 310 ℃, sheath gas flow rate 35 arb, aux
gas flow rate 10 arb, spray voltage 3.60 kV, S-lens
RF level 50.0.
S
O
O
NH
2
H
2
N
HN
HCL
S
O
O
NH
2
H
2
N
Determination of 4-Sulfonamidophenylhydrazine and Sulfonamide in Diarylpyrazole Derivatives by LC-MS
993

Figure 1: Representative mass spectra of
4-sulfonamidophenylhydrazine and sulfonamide.
2.5 Standard and Sample Preparation
The separate stock standard solutions were prepared
by weighing an accurately amount of about 10 mg of
4-sulfonamidophenylhydrazine and sulfonamide,
and transferred them into a 100 mL volumetric flask
individually, the volume was made up to the mark
with methanol. The mixed stock standard solution
was prepared by diluting 1 mL of
4-sulfonamidophenylhydrazine standard solution
and 1 mL of sulfonamide stock standard solution to
100 mL with methanol. Take an appropriate amount
of the mixed stock standard solution, and gradually
dilute it with methanol to make a solution containing
5 ng/mL of 4-sulfonamidophenylhydrazine and 5
ng/mL of sulfonamide, as the standard solution.
The sample solution was prepared by dissolving
appropriate amount of diarylpyrazole derivatives in
methanol to make a solution containing 2 mg/mL.
3 METHOD VALIDATION
3.1 Specificity
The specificity of the method was demonstrated by
injecting the blank and the reference solution. The
results showed that the retention time of
4-sulfonamidophenylhydrazine was 2.84 min, and
the retention time of sulfonamide was 4.37 min
(Figure 2). The blank chromatogram showed that no
interference was observed at the retention times of
4-sulfonamidophenylhydrazine and sulfonamide.
Figure 2: Specificiy of the method.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
994

3.2 Solution Stability
The stability of 4-sulfonamidophenylhydrazine and
sulfonamide was checked by keeping the standard
solution in an autosampler for 12h and observing the
variations in their peak areas at every 1h. The RSDs
of the peak areas of 4-sulfonamidophenylhydrazine
and sulfonamide were 5.28% and 3.39%,
respectively. From the stability results, we found
that 4-sulfonamidophenylhydrazine and sulfonamide
were stable up to 12h.
3.3 Determination of LOD and LOQ
Take the standard solution and dilute it step by step
with methanol until the signal-to-noise ratio S/N is
close to 10 and 3 as the limit of quantification and
detection of 4-sulfonamidophenylhydrazine and
sulfonamide. The limits of quantification for
4-sulfonamidophenylhydrazine and sulfonamide
were 0.4915 ng/mL and 0.5079 ng/mL, respectively.
The detection limits of
4-sulfonamidophenylhydrazine and sulfonamide
were 0.1474 ng/mL and 0.1524 ng/mL, respectively.
3.4 Linearity
The linearity stock solution was prepared by diluting
5 mL of the mixed stock standard solution to 100
mL with methanol. The linearity test solutions were
prepared from the linearity stock solution at seven
concentration levels from LOQ (0.25 ppm) to 300%
(7.5 ppm) of the specification concentrantion (2.5
ppm). The calibration curve was obtained by
drawing the graph between the peak areas and
concentration of 4-sulfonamidophenylhydrazine and
sulfonamide at 0.25, 0.5, 1, 1.5, 2.5, 5 and 7.5 ppm.
The slope, intercept, and correlation coefficient
values were derived from linear least squares
regression analysis. The correlation coefficient
obtained in each case was >0.9998. The
corresponding linearity data is presented in Table 3.
The results indicated that an excellent correlation
existed between the peak areas and the
concentrations of 4-sulfonamidophenylhydrazine
and sulfonamide.
Table 3: Results of linearity for 4-sulfonamidophenylhydrazine and sulfonamide.
Level
(ppm)
4-Sulfonamido-
-phenylhydrazine
Sulfonamide
Conc.
(ng/mL)
Peak area
Conc.
(ng/mL)
Peak area
LOQ 0.4915 53731.87 0.5079 108697.69
0.5 0.9829 112075.22 1.0159 208294.14
1 1.9659 249253.89 2.0318 413800.80
1.5 2.9488 367906.96 3.0477 654523.96
2.5 4.9147 633583.15 5.0795 1069378.87
5 9.8294 1238938.63 10.1590 2098273.04
7 14.7441 1878797.94 15.2384 3113619.89
Correlation
0.9999 0.9999
Slope 127733 204377
Intercept 6917.3 12610
3.5 Recovery studies
A study of accuracy of
4-sulfonamidophenylhydrazine and sulfonamide
from spiked samples of test preparation was
conducted. Samples were prepared in triplicate at
LOQ level, 100% and 150% of the specification
concentrations, i.e 0.25, 2.5 and 5 ppm by spiking
test preparation. The mean recovery of
4-sulfonamidophenylhydrazine and sulfonamide at
mentioned concentration level was reported in the
Table 4. The recoveries of
4-sulfonamidophenylhydrazine and sulfonamide at
three levels were in the range of 80% to 120% and
relative standard deviations were not more than
10.0%.
Determination of 4-Sulfonamidophenylhydrazine and Sulfonamide in Diarylpyrazole Derivatives by LC-MS
995

Table 4: Accuracy of 4-sulfonamidophenylhydrazine and sulfonamide
Sample Name
Recovery (%)
4-sulfonamido-
-phenylhydrazine
Sulfonamide
LOQ spiked sample-1
96.46 109.19
LOQ spiked sample-2
102.12 120.06
LOQ spiked sample-3
99.33 117.28
100% spiked sample-1
105.33 107.41
100% spiked sample-2
113.12 101.43
100% spiked sample-3
114.59 105.16
150% spiked sample-1
111.68 108.45
150% spiked sample-2
109.24 94.70
150% spiked sample-3
110.81 96.70
Mean recovery (%)
106.96 106.71
RSD (%)
6.03 7.94
3.6 Precision
3.6.1 System Precision
The system precision was checked by calculating the
RSD of six areas of 4-sulfonamidophenylhydrazine
and sulfonamide by injecting the same standard
solution. The RSDs of six areas of
4-sulfonamidophenylhydrazine and sulfonamide
were 2.55% and 2.03% respectively.
3.6.2 Method Precision
The precision of the method was evaluated through
repeatability and intermediate precision.
Repeatability was checked by calculating the RSD
of six replicate determinations by injecting six
freshly prepared solutions containing 2.5 ppm each
of 4-sulfonamidophenylhydrazine and sulfonamide
on the same day. The same experiments were done
on different days by different people to evaluate the
intermediate precision. As reported in Table 5, the
data confirmed adequate precision of the developed
method.
Table 5: Method precision of 4-sulfonamidophenylhydrazine and sulfonamide at 2.5 ppm in terms of percentage contents.
Injection
4-sulfonamido-
-phenylhydrazine
Sulfonamide
Repeata-
-bility
Intermediate
precision
Repeata-
-bility
Intermediate
precision
1 3.10E-04 2.95E-04 2.84E-04 2.67E-04
2 2.92E-04 2.86E-04 2.80E-04 2.64E-04
3 2.88E-04 2.85E-04 2.82E-04 2.78E-04
4 2.92E-04 2.91E-04 2.79E-04 2.58E-04
5 2.81E-04 2.70E-04 2.80E-04 2.49E-04
6 2.82E-04 2.68E-04 2.80E-04 2.49E-04
Mean
(%)
2.91E-04 2.82E-04 2.81E-04 2.61E-04
RSD
(%)
3.64 3.92 0.65 4.31
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
996

4 DISCUSSION
Diarylpyrazole derivatives have good solubility in
methanol. Sulfonamide and
4-sulfonamidophenylhydrazine are slightly soluble
in methanol. Considering the large concentration of
principal components and small impurity
concentration, methanol is finally determined as the
solvent.
In order to control the lower limit level of
genotoxic impurities, this study selected a mass
spectrometer for detection. Since a higher
concentration of sample solution may contaminate
the MS detector, the chromatographic method that is
considered to be established should be able to
achieve good separation of impurities peaks and
principal component peaks, so that the principal
component peaks do not enter the MS detector by
switching the valve. The structure of sulfonamides
and 4-sulfonamidophenylhydrazine contains one
benzene ring, while diarylpyrazole derivatives
contain two, the test results show that the phenyl
column which has a special selectivity for aromatic
compounds could achieve better separation of
sulfonamides, 4-sulfonamidophenylhydrazine and
diarylpyrazole derivatives.
The mass detector requires the use of volatile
mobile phase additives. Ammonium acetate was
used to optimize the peak shape of
4-sulfonamidophenylhydrazine and sulfonamide.
With adjusting the pH value to 4.0 to achieved the
baseline separation of
4-sulfonamidophenylhydrazine and sulfonamide. By
adjusting the organic proportion and gradient
elution, the retention time of diarylpyrazole
derivatives is 5 minutes later so that the switch valve
could control the main peak not to flow into the
mass spectrometer, while ensuring that the main
peak can be completely eluted every time to avoid
affecting the next sample.
5 CONCLUSIONS
A validated LC-MS analytical method has been
developed for the determination of
4-sulfonamidophenylhydrazine and sulfonamide in
diarylpyrazole derivatives. The proposed method
was simple, accurate, precise, specific and suitable
to use for the routine analysis of
4-sulfonamidophenylhydrazine and sulfonamide in
diarylpyrazole derivatives.
ACKNOWLEDGMENTS
We would like to express our gratitude to all those
who helped us in researching and writing this
project. Thanks to the colleagues in the synthesis
department for their hard work. Thanks are due to
the colleagues from the science and technology
information department for providing access to the
literature data.
REFERENCES
Baran W, Adamek E, Ziemianska J, et al. Effects of the
presence of sulfonamides in the environment and their
influence on human health [J]. J. Hazard. Mater., 2011,
196 (1): 1-15.
Bekhit A A, Tarek Abdel-Aziem. Design, synthesis and
biological evaluation of some pyrazole derivatives as
anti-inflammatory-antimicrobial agents [J]. Bioorg.
Med. Chem. Lett., 2004, 12: 1935-1945.
Ghofrani H A, Grimminger F. Soluble guanylate cyclase
stimulation: an emerging option in pulmonary
hypertension [J]. Eur. Respir. Rev., 2009, 18(111):
35-41.
Gogas H, Mansi J L. The anthrapyrazoles [J]. Cancer
Treatment Reviews, 1995, 21: 541-552.
Gong Q, Zhong X W, Tian J, et al. Discussion on
Requirements of Starting Materials in Chemistry
Synthesized Drug Substances [J]. Chin. Pharm. Aff.,
2019, 33(8): 864-870.
Katoch-Rouse R, Pavlova O A, Tara Caulder, et al.
Synthesis, Structure-Activity Relationship, and
Evaluation of SR141716 Analogues: Development of
Central Cannabinoid Receptor Ligands with Lower
Lipophilicity [J]. J. Med. Chem., 2003, 46: 642-645.
Liu X H, Song B A, Li B. Synthesis and Fungicidal
Activity of Novel 3,5-Diarylpyrazole Derivatives [J].
Chin. J. Appl. Chem., 2007, 24(7): 835-837.
Pathak R B, Chovatia P T, Parekh H H. Synthesis,
antitubercular and antimicrobial evaluation of
3-(4-chlorphenyl)-4-substituted pyrazole derivate [J].
Bioorg. Med. Chem. Lett., 2012, 22: 5129-5133.
Penning T D, Talley J J, Bertenshaw S R, et al. Synthesis
and Biological Evaluation of the 1,5-Diarylpyrazole
Class of Cyclooxygenase-2 Inhibitors: Identification
of
4-[5-(4-Methylphenyl)-3-(trifluoromethyl)-1H-pyrazol
-1-yl] benzenesulfonamide (SC-58635, Celecoxib) [J].
J. Med. Chem., 1997,40: 1347-1365.
Rajput M, Patel N, Chotaliya U, et al. Determination of
genotoxic impurity by chromatographic method [J].
Pharma Sci. Monit., 2017,8(2): 24-31.
Reddy A V B, Jaafar J, Umar K, et al. Identification,
control strategies, and analytical approaches for the
determination of potential genotoxic impurities in
pharmaceuticals: A comprehensive review [J]. J. Sep.
Sci., 2015, 00: 1-16.
Determination of 4-Sulfonamidophenylhydrazine and Sulfonamide in Diarylpyrazole Derivatives by LC-MS
997

Selvam C, Jachak S M, Thilagavathi R, et al. Design,
synthesis, biological evaluation and molecular
docking of curcumin analogues as antioxidant,
cyclooxygenase inhibitory and anti-inflammatory
agents [J]. Bioorg. Med. Chem. Lett. , 2005, 15:
1793-1797.
Stauffer S R, Coletta C J, Tedesco R, et al. Pyrazole
Ligands: Structure-Affinity/Activity Relationships and
Estrogen Receptor-α-Selective Agonists [J]. J. Med.
Chem., 2000, 43:4934-4947.
Szekely G, Sousa M C, Gil M, et al. Genotoxic impurities
in pharmaceutical manufacturing: Sources, regulations,
and mitigation [J]. Chem Rev, 2015, 115(16):
8182-8229.
Tang J, Xiang G Y. Synthesis and COX-2 inbibitory
activities of 1,5-diaryl pyrazole derivatives [J]. Chin. J.
Med. Chem., 2008, 18(1): 6-10.
Wang J H, Wang C Y, Chen B, et al. Synthesis and
structure activity relationship of anthrapyrazoles
derivatives [J]. J. Henan Univ., Med. Sci., 2014, 33(3):
164-166.
Yu C M, Shi J Y, Pan L L, et al. The Study of Synthesis for
4-Sulfonamidophenylhydrazine Hydrochloride [J].
Zhejiang Chem. Ind., 2015, 46(5): 19-21.
Zhang L, Wang J, Li W B, et al. Advances in Research on
Activities of Pyrazole Derivatives [J]. World Notes on
Antibiot., 2014, 35(4): 149-153, 168.
Zhong Chen, Zhong-Chang Wang, Xiao-Qiang Yan, et al.
Design, synthesis, biological evaluation and molecular
modeling of dihydropyrazole sulfonamide derivatives
as potential COX-1/COX-2 inhibitors [J]. Bioorg.
Med. Chem. Lett., 2015, 25: 1947-1951.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
998
