
PRECLINICAL TESTING OF A NEW VENOUS VALVE
Laura-Lee Farrell and David N. Ku
Georgia Institute of Technology, Atlanta, GA 30332-0405, USA
Keywords: Vein, venous valves, thrombosis, patency.
Abstract: Venous valvular incompetency is a debilitating disease affecting millions of patients. Unfortunately, the
current physiologic and surgical treatments are prone to the extreme risk of post-operative thrombosis. A
new design for venous valves has been proposed using biomimicry. The medical device has the shape of a
natural valve with sufficient elasticity to maintain patency and competency in the leg veins. The venous
valve was tested for patency, competency, cyclic fatigue, compressibility, and thrombogenicity. Patency is
maintained with a low opening pressure of less than 3 mmHg. Competency is maintained with
backpressures exceeding 300 mmHg. The valve is fatigue resistant to over ¼ million cycles. The valve can
maintain its integrity when compressed in a stent and deployed without tilting or mal-alignment. Little
thrombus forms on the valve with perfusion of whole blood under pulsatile flow conditions. The pre-
clinical tests demonstrate efficacy as a new venous valve for treatment of chronic venous insufficiency.
1 BACKGROUND
Venous disease will affect 1-3% of the western
world at some point in their lives, yet there are few
effective treatments for the venous system. One such
disease is chronic venous insufficiency (CVI), a
painful and debilitating illness that affects the
superficial and deep vein valves of the legs. When
the valves become incompetent they allow reflux
and subsequent pooling of blood. Symptoms include
swelling, edema, pain, itching, varicose veins, skin
discoloration, ulceration and limb loss. Post-
thrombotic damage within the deep veins is the most
significant cause of CVI, reported as high as 95%.
Current clinical therapies are only moderately
effective; and therefore, the need for a better
solution remains.
Surgical treatment for CVI is avoided due to
lack of accurate surgical technology and the extreme
risk of post-operative thrombosis. Valvuloplasty is
extensively time consuming and reserved for
patients with a congenital absence of functional
valves and severe cases of CVI. This surgical
procedure involves a venotomy, where the valve
cusps are plicated 20-25%. A singular valvuloplasty
is usually sufficient to correct CVI except in systems
that include occlusion of the femoral or popliteal
vein, or absence/incompetence of the
communicating leg veins.
Previous prosthetic vein valves have
experienced complications due to either in vivo
thrombosis, in-growth, foreign body reaction, or
prosthetic disorientation (tilting). The umbilical vein
valves and pellethane valves by Hill et al. failed due
to thrombotic occlusion (Hill et al, 1985). Two-
thirds of the autogenous venous valves by
Rosenbloom et al. failed from complications arising
to thrombosis (Rosenbloom et al, 1988). The
mechanical valves by Taheri et al. failed after three
months due to severe thrombotic occlusion (Taheri
and Schultz, 1995). Partial thrombosis appeared in
the valve cusps of the polyetherurethane valves by
Uflacker (Uflacker, 1993). Thrombosis occurred
immediately after deployment of the gluteraldehyde-
fixed bovine external jugular by Gomez-Jorge et al.
(Gomez-Jorge et al, 2000). Glutaraldehyde-
preserved bovine jugular valve-bearing venous
xenograft show thrombosis by histology (de Borst et
al, 2003).
A second cause of failure is hyperplasia.
Biocompatibility was the primary concern for Taheri
et al. and Gomez-Jorge et al. The two-year non-
patent mechanical valves by Taheri et al. failed due
to dense in-growth of intimal hyperplasia, which
rendered the valves functionless. The
gluteraldehyde-fixed bovine external jugular by
Gomez-Jorge et al. produced a granulomatous
response and foreign body reaction (Gomez-Jorge et
30
Farrell L. and N. Ku D. (2008).
PRECLINICAL TESTING OF A NEW VENOUS VALVE.
In Proceedings of the First International Conference on Biomedical Electronics and Devices, pages 30-35
DOI: 10.5220/0001050000300035
Copyright
c
SciTePress

al, 2000). Between 2000 and 2005 Pavcnik et al
reported on a stent-based porcine small intestine
submucosa prosthesis (Pavcnik et al, 2005); all
failed valves were the result of prosthesis tilting.
Overall, eight out of ten of the reported valve
designs experienced complications due to
thrombosis.
A new design for venous valves has been
proposed using biomimicry. The medical device
has the shape of a natural valve with sufficient
elasticity to maintain patency and competency in the
leg veins. This paper describes the pre-clinical
verification and validation testing of this new venous
valve.
2 METHODS
The venous devices were subjected to a battery of
tests to demonstrate sufficient function as a one-way
valve, propensity for thrombosis, and suitability for
minimally invasive delivery. The new “GT” vein
valve prosthetic is presented in Figure 1.
2.1 Pulsatile Flow System
This pulsatile system was designed to mimic the
physiologic flow conditions present in the lower
extremity venous system. During normal walking,
compression occurs about 40 times a minute (0.67
Hz). Fresh, whole, porcine blood with heparin (6.0 ±
0.2 U/mL) was transferred into a blood donor
collection bag. The collection bag was raised 30 cm
above the test section, rotated on an orbital mixer,
and attached to 90 cm of vinyl tubing, followed by a
3-way valve, a pressure tap and the test section.
Downstream of the test section, a 50 cm segment of
tubing (3.5 mm inner diameter) was passed through
a pulsatile pump rotating at a frequency of 0.75 Hz.
Pressure upstream of the vein valve was recorded
with a pressure transducer (Harvard Apparatus,
South Natick, MA) and the flow rate was calculated
from measurements with a graduated cylinder and a
stopwatch. The experiment proceeded until flow
cessation by occlusion or the contents of the fluid
reservoir were emptied.
Figure 1: GT vein valve. Upper left, isometric view. Upper
right, cross sectional view. Lower, downstream view.
2.2 Test Section
The test section included a vein valve, a flexible
venous-like tube, and suture material. The valves
were manufactured according to the procedure
outlined in reference (Sathe, 2007). The valve
material was made from a PVA hydrogel
biomaterial. The valve was inserted into the flexible
tube and tightly tied in place to prevent blood from
passing between the valve and the vessel wall. The
flexible tube was further attached to the vinyl tubing
by securing it with suture.
2.3 Dacron Lined Valve
A Dacron-lined valve acted as the positive control.
The lining was constructed from a commercially
available cardiovascular Dacron patch often used
clinically of approximately 14 mm ± 1 mm by 9 mm
± 0.5 mm, which was then sutured to the GT valve.
One stitch was placed on each Dacron piece on the
upstream side, these sutures held the Dacron against
the GT valve.
2.4 Pressure Tests
A syringe was attached to a three-way valve with the
test section and the pressure transducer (Harvard
Apparatus, South Natick, MA); downstream the test
section was open to atmosphere. Pressure was
applied with the syringe and read upstream of the
test section. For opening pressure, the prosthetic
vein valve was orientated with the distal end closest
PRECLINICAL TESTING OF A NEW VENOUS VALVE
31

to the syringe, and the proximal end facing ambient
atmosphere. For backpressure, the prosthetic valve
was reversed in orientation.
2.5 Thrombosis of Whole Blood
Whole blood samples were harvested from pigs and
quickly anti-coagulated with porcine heparin to a
final concentration of 6.0 ± 0.2 U/mL. The samples
were mixed with a nutating rocker at approximately
42 rpm for 15 minutes prior to the experiment.
Experiments were completed within eight hours of
harvesting the blood and conducted at room
temperature.
2.6 Histology
Samples were fixed in 10% formalin (VWR
International, West Chester, PA) for at least 72
hours. Samples were processed and embedded in
paraffin. Deformation of the samples during
processing was expected to be between 30 to 50%.
Samples were cut into 5-micron thin circular cross-
sections, oriented perpendicular to flow. Eight
sections from orifice areas were collected from each
sample. Alternating samples were stained with
Haematoxylin and Eosin stain (H&E), and Carstair’s
stain (specific for platelets). Sections were analyzed
microscopically using a Nikon E600 microscope, a
digital camera and Q-capture software.
2.7 Flat Compression
The valves were evaluated for plastic deformation
with respect to compression time. Initially, they
were evaluated for opening pressure and
backpressure conditions. At periodic time points the
valves were allowed to expand and were re-
evaluated for opening pressure and backpressure
conditions.
2.8 Radial Compression
The GT valves were inserted into balloon
expandable Palmaz stents, 10mm diameter and 20-
25mm in length, (Cordis Endovascular, Miami, FL;
and IntraTherapeutics, St. Paul, MN), and sutured
into place. The valves were evaluated for opening
pressure and backpressure.
2.9 In Vivo Placement
Placement inside an actual vein has been
problematic for some previous designs. The valves
might tilt or dislodge in the vein. Thus, our valves
were surgically placed in the correct anatomic
position in animals. The external jugular veins
(EJV) were exposed on four previously deceased,
2.5 year old, 50-60 Kg Dorset ewe sheep. A vertical
incision was performed on the EJV and the
prosthetic was placed inside the vein. The vessel
diameter was measured and the prosthetic valve was
manipulated to evaluate potential misplacement.
This procedure was repeated on the iliac veins.
3 RESULTS
The GT vein valve was evaluated for patency,
competency and cyclic life (Sathe, 2006). The valve
withstood 300 mm Hg of backpressure with less
than 0.3 mL leakage per minute, demonstrated a
burst pressure of 530 ± 10 mm Hg , opened with a
pressure gradient as low as 2.0 ± 0.5 mm Hg. The
patency and competency endpoints were statistically
unchanged after 500,000 cycles of cyclic testing.
3.1 Thrombosis from Pulsatile Blood
Flow
Blood was perfused through five GT vein valves; a
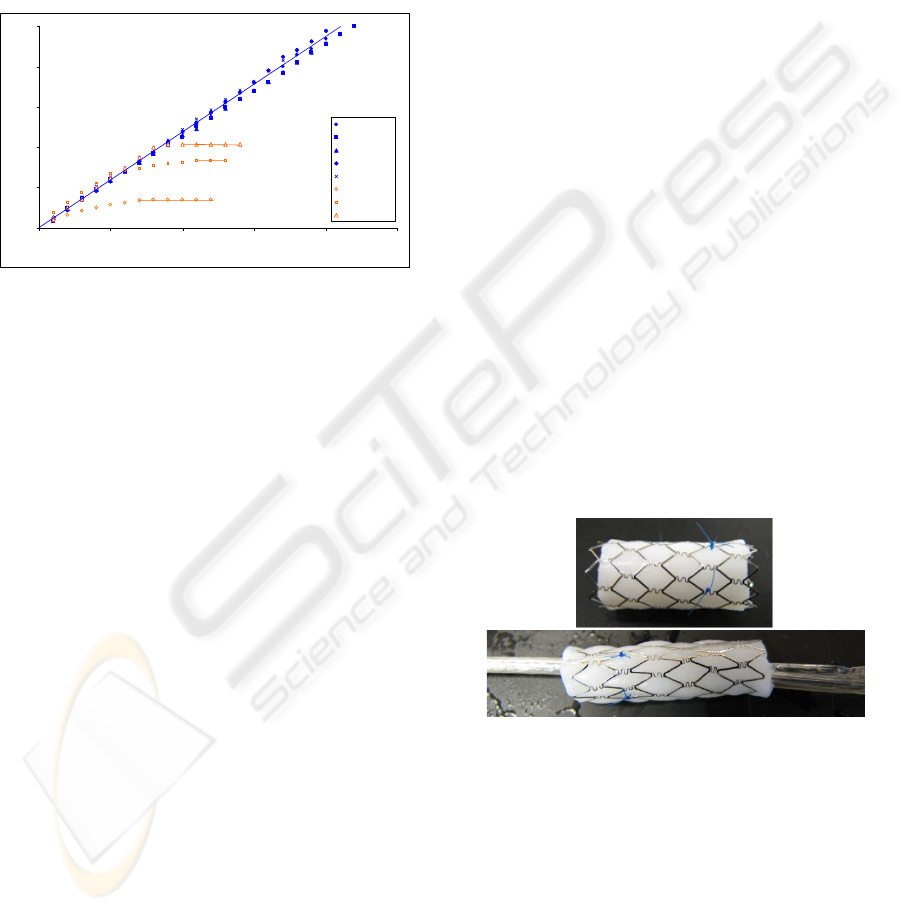
graphical representation is shown in Figure 2. All
five valves remained patent after 20 minutes of
blood flow without significant flow rate
deterioration. The average blood flow rate was 11.8
± 0.4 mL/min. The upstream pressure fluctuated
between 15 – 21 mmHg. Once the system was
exhausted of blood, the pressure dropped off to just
above 10 mmHg. When the flow system depleted
the blood reservoir the roller pump tried to pull
blood through the valve, the upstream and
downstream sections of the valve would collapse
due to the negative pressure. There was no gross
thrombus visible on any of the valve leaflets. The
leaflets remained functional and the valves remained
competent against backpressure.
The Dacron-lined valves initially produced the
same velocity profiles as the GT valves; though,
they did not remain patent for the experiment, but
rather occluded completely. The flow rate reduced
after two to eight minutes into the perfusion. On
average, the Dacron lined valves occluded after 6 ±
3.6 min of perfusion. The upstream pressure
fluctuation prior to occlusion was between 15 – 21
mmHg, and after occlusion, the pressure was
constant at 24 ± 1mmHg. The frequency of
occlusion for the Dacron lined valves in this assay
was significant to p<0.02. With occlusion, the
BIODEVICES 2008 - International Conference on Biomedical Electronics and Devices
32
flexible tube collapsed violently instead of the valve
reopening. Thus, the system was a severe
demonstration of the adherent nature of the
occluding thrombus. The Dacron valves were
visually inspected at the end of the experiment. The
polyester fibers were covered with red blood and
were visibly matted down. After occlusion, some
red clot remained in the lumen of the tubes. The GT
valves and Dacron lined valves were preserved for
histological analysis.
0
50
100
150
200
250
0 5 10 15 20 25
Time (min)
Volume (mL)
PVA Valve 1
PVA Valve 2
PVA Valve 3
PVA Valve 4
PVA Valve 5
Dacron Valve 1
Dacron Valve 2
Dacron Valve 3
Figure 2: Perfused blood volume over time in the GT and
Dacron-lined valves. The GT valves produced a constant
flow rate; whereas, the Dacron lined valves produced a
gradual cessation of flow.
Histology was performed on both the GT and
Dacron lined valves to identify cell accumulation
and the cause for cessation of flow. The histology
stains used were Haematoxylin and Eosin stain
(H&E), and Carstair’s stain (specific for platelets).
The PVA material is represented as pink in the H&E
stain and a faint blue-grey in the Carstair’s stain.
With regard to the Dacron lined valve slides, the
gray circular structures represented the Dacron
fibers. The red debris located between the Dacron
leaflets represented the cellular material that was
preventing blood from passing through the leaflets in
the in vitro model. Further analysis with Carstair’s
stain revealed that platelet aggregation with fibrin
strands was a key component in the red debris. The
presence of platelets on the Dacron leaflets, and the
complete absence of platelets on the GT valves
confirmed that the in vitro blood flow assay had the
potential to thrombose, yet the GT valves do not
exhibit any thrombosis or clot in this system.
3.2 Plastic Deformation – Flat
Compression.
Valves were subjected to flat compression. Prior to
compression the valves demonstrated an opening
pressure of 3 mm Hg ± 1 mm Hg, and a
backpressure of at least 100 mm Hg. Subsequently at
2 hrs, 4 hrs and 6 hrs after compression the valves
exhibited an opening pressure of 3 mm Hg and
maintained competency with a backpressure of 100
mm Hg.
3.3 Radial Compression
The average initial outside diameter of the valve-
stent system was 8.8 mm ± 0.1 mm. Prior to
compression exposure the valves demonstrated an
opening pressure of 3 mm Hg ± 1 mm Hg, and a
minimum backpressure of 100 mm Hg. The average
compressed outside diameter of the valve-stent
system was 6.5 mm ± 0.1 mm. They were
compressed for 1hr and subsequently expanded,
shown in Figure 3. Visually the expanded valves
retained their original configuration. The valves
exhibited an opening pressure of 4 mm Hg ± 1 mm
Hg and withstood a backpressure of 100 mm Hg.
The average expanded outside diameter of the valve-
stent system was 9.5 mm ± 0.5 mm. All valves met
the original design criteria of opening pressure
below 5 mm Hg and a backpressure up to 100 mm
Hg.
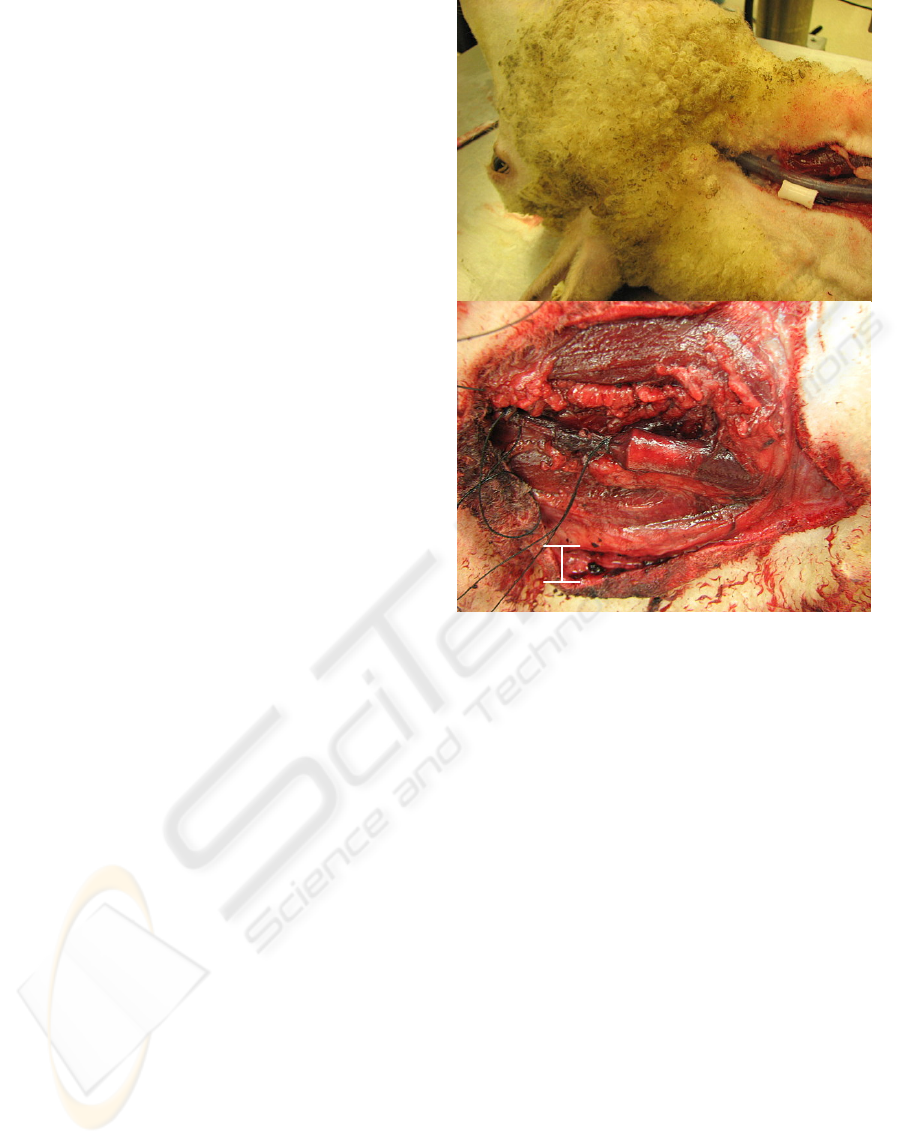
3.4 In Vivo Placement
The prosthetic was positioned inside the external
jugular veins and iliac veins of four sheep as
depicted in Figure 4. The 10 mm prosthetic vein
valve was of appropriate size for the EJV of sheep.
Figure 3: Above, Genesis Palmaz Stent (Cordis) with GT
vein valve. Below, radially compressed valve and stent on
a balloon catheter.
The 10 mm prosthetic vein valve was too small
for the iliac vein. Vigorous manipulation of the
prosthetic in situ did not cause any misplacement,
tilting, or orientation problems. Tilting was of no
concern due to the long profile of the prosthetic. A
suite of valve sizes ranging from 10mm to 4 mm in 2
mm increments were created to account for varying
vessel sizes, as seen in the iliac of sheep.
PRECLINICAL TESTING OF A NEW VENOUS VALVE
33
4 DISCUSSION
Evaluating the thrombotic potential of a prosthetic
vein valve in an in vitro set-up is a novel process, as
the thrombotic potential is typically evaluated in an
animal model. Animal studies require the long
process of approval from animal care and use
committees, the trials are costly, the study itself is
time consuming, and animal lives are sacrificed. In
vivo models are necessary to determine the
biocompatibility of the prosthetic device, and an
important step towards clinical trials; yet using an in
vitro thrombosis model provides an appropriate
intermediate step between valve development and
expensive in vivo studies.
The GT venous valve demonstrates low
thrombus formation in the whole blood perfusion
system, as it remained patent after 20 minutes of
perfusion with no adherent platelets. In contrast,
the Dacron valves occluded after 6 ± 3.6 min of
perfusion. Histology revealed adherent fibrin, RBCs
and platelet thrombus under histological analysis.
The time of occlusion for the Dacron lined valves in
this assay was significantly shorter than the GT
valves (p<0.02).
When designing an in vitro model it is most
relevant for the model to be as close to physiologic
conditions as possible. The in vitro model perfuses
whole porcine blood through a prosthetic vein valve.
The pulsatile frequency of the system, 0.75Hz,
approximates the normal walking cadence of an
adult. A potential limitation to this in vitro set-up is
that the flow through the prosthetic valves was 11.8
± 0.4 mL/min, yet the blood flow through the
femoral vein is around 70 mL/min. The flow was
lower than physiologically observed valves because
the frequency and collection time were selected, but
the tubing diameter was restricted. The tubing
diameter could not be increased to reduce flow as it
was limited to the pulsatile pump tubing
specifications. Even though platelet adhesion in a
stenosis happens at high velocities, vein thrombosis
typically is thought to occur at low velocities.
Therefore modeling a low flow rate may be more
appropriate, since it is a worse-case scenario.
For instance, when one sits for a long period of
time on a transatlantic flight and the calf pump is not
actively engaged, the blood is traveling at a lower
velocity back to the heart.
Figure 4: Above, GT valve positioned beside right
external jugular vein. Below, GT valve implanted into
right external jugular vein.
The future of medical implants lies in
percutaneous devices; therefore, to create a
marketable and less invasive implant, a percutaneous
delivery system has been designed for the GT vein
valve. An appropriate delivery route may be from
the external jugular vein down through the heart to
the femoral or iliac vein. Reduction of the crimped
valve profile may be achieved by decreasing the
thickness of the cylindrical supporting material.
Future improvements could include incorporation of
antithrombotics or other eluting drugs into the valve
to limit thrombosis, inflammation or foreign body
response mechanisms. Due to the low in vitro
thrombotic potential and the successful previous
clinical use of the material as a medical implant
material, clinical trials may be considered.
Given the successful implementation of pre-
procedure crimping of percutaneous heart valves
(Edwards Life Sciences), a similar technique was
pursued for the GT vein valve to allow it to be
compressed within 6 hours of implantation. The
portable stent crimper makes this possible.
Evaluating the thrombotic potential of a prosthetic
1 mm
BIODEVICES 2008 - International Conference on Biomedical Electronics and Devices
34

vein valve in an in vitro blood set-up is a novel
process. The most common practice to test the
thrombotic potential is in an animal model, where
eight out of ten studies reviewed failed due to in vivo
thrombosis. The two most successful vein valve
studies use acellular tissues: the SG-BVV was
constructed from porcine small intestine submucosa
(SIS), and the PVVB used gluteraldehyde-preserved
bovine jugular valves (Moll, 2003), (Gale et al,
2004). The GT vein valve provides several
advantages over SIS and gluteraldehyde-preserved
bovine jugular valves. Zoonosis from animal tissue
prosthetics is possible and the use of animal derived
prosthetics may be culturally or religiously
controversial, therefore a synthetic material would
alleviate these concerns. The PVVBs are fixed with
gluteraldehyde, which is a toxic substance that will
prevent cells from integrating into the material in
vivo. This gluteraldehyde preservation process will
cause a limited cyclic life due to the cross-linking of
the collagen fibers, and ongoing biocompatibility
issues due to the gluteraldehyde toxicity. The SIS
tissue appears to be an appropriate material for vein
valve prosthetics with regards to its
biocompatibility. However, despite revisions to the
SIS vein valve, the SG-BVV continues to experience
in vivo tilting. Tilting is not an issue with the GT
vein valve because of the long axial dimension. In
addition, GT vein valve can be mass-produced and
the design is easily modified. This is unlike acellular
tissues which require extensive tissue preparation
and processing times and modification of the tissue
valve design could create concerns regarding
suturing locations and tissue-to-stent attachment
sites.
Another feature of the new GT Valves is that
they may be processed to include embedded drugs,
which could promote cell growth and/or reduce
thrombus formation. It has superior biocompatibility
and structural integrity, may be mass-produced, and
has the potential to utilize new drug delivery
technologies.
Providing relief to chronic venous insufficiency
is a worthwhile pursuit as patients experience
swelling, edema, pain, itching, varicose veins, skin
discoloration, ulceration and limb loss. Current
clinical therapies are only modestly effective; and
therefore, a prosthetic vein valve may provide a cure
for this debilitating disease. With successful animal
and human trials this valve could provide a useful
therapy the 7 million people suffering from chronic
venous insufficiency. The GT valve exhibits
excellent flow, full competency, fatigue-resistance,
low-thrombogenicity, material flexibility, and in situ
placement consistency.
REFERENCES
de Borst, G.J., et al., A percutaneous approach to deep
venous valve insufficiency with a new self-expanding
venous frame valve. Journal Of Endovascular Therapy,
2003. 10(2): p. 341-349.
Gale, S.S., et al., Percutaneous venous valve
bioprosthesis: initial observations. Vascular And
Endovascular Surgery, 2004. 38(3): p. 221-224.
Gomez-Jorge, J., A.C. Venbrux, and C. Magee,
Percutaneous deployment of a valved bovine jugular
vein in the swine venous system: a potential treatment
for venous insufficiency. Journal Of Vascular And
Interventional Radiology: JVIR, 2000. 11(7): p. 931-
936.
Hill, R., et al., Development of a prosthetic venous valve.
Journal of biomedical materials research., 1985. 19(7):
p. 827.
Moll, F. Venous Valves for Chronic Venous Insufficiency.
in Vascular and Endovascular Controversies. 2003.
London, UK.
Pavcnik, D., et al., Significance of spatial orientation of
percutaneously placed bioprosthetic venous valves in
an ovine model. Journal Of Vascular And
Interventional Radiology, 2005. 16(11): p. 1511-1516.
Rosenbloom, M.S., et al., Early experimental experience
with a surgically created, totally autogenous venous
valve: a preliminary report. Journal of vascular
surgery, 1988. 7(5): p. 642.
Sathe, R.D. and D.N. Ku, Design and Development of a
Novel Implantable Prosthetic Vein Valve. J Med
Devices, 2007. 1: p. 105-112.
Taheri, S.A. and R.O. Schultz, Experimental prosthetic
vein valve. Long-term results. Angiology., 1995.
46(4): p. 299.
Uflacker, R. Percutaneously introduced artificial venous
valve: Experimental use in pigs. in The 1993 Annual
Meeting of the Western Angiographic & Interventional
Society. 1993. Portland, OR.
PRECLINICAL TESTING OF A NEW VENOUS VALVE
35
